1 Global Pre-Filled Heparin Lock Syringe Market Outlook
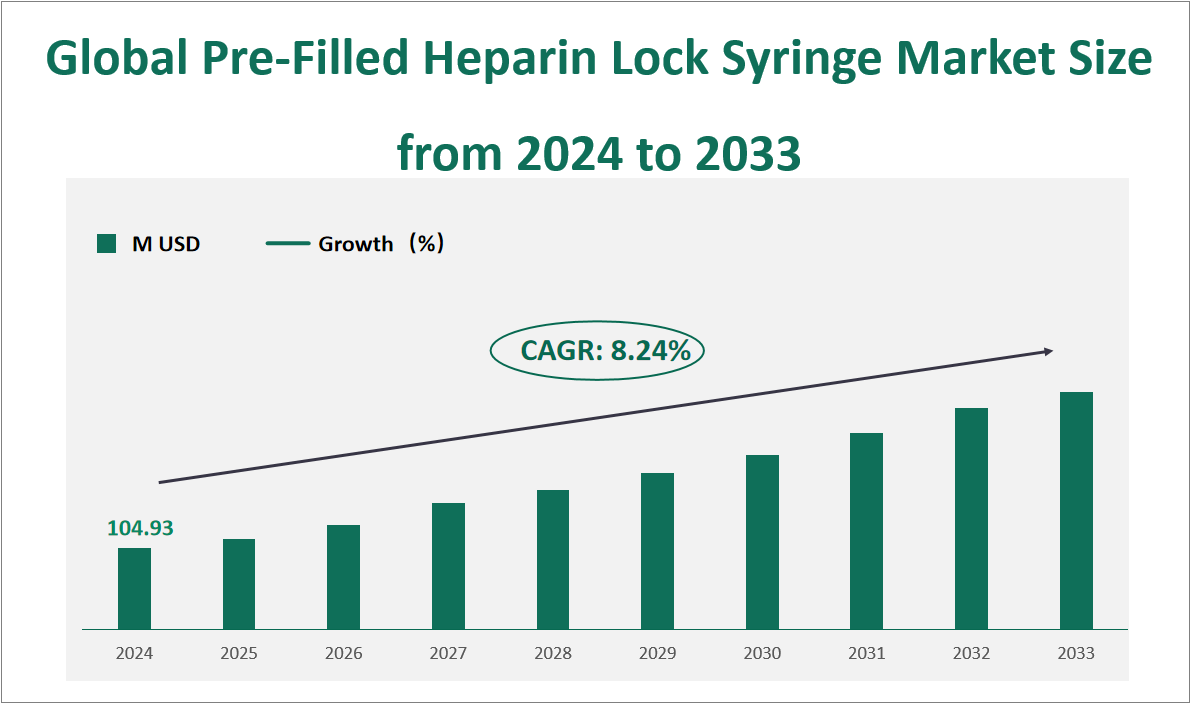
The global Pre-Filled Heparin Lock Syringe market is projected to exhibit substantial growth in the coming years, with a CAGR of 8.24% from 2024 to 2033, reaching a total market size of $104.93 million USD in 2024. A pre-filled heparin lock syringe is a specialized medical device containing a heparin solution designed to maintain the patency of intravenous catheters and reduce the risk of blood clots. These syringes are widely used in hospitals, outpatient clinics, homecare settings, and pharmaceutical companies to ensure the safe and efficient administration of heparin, an anticoagulant. The market for these syringes is segmented based on capacity, with 3ml, 5ml, and 10ml sizes being the most common. Among these, the 5ml syringe size holds the largest market share, driven by its versatility and suitability for various medical applications.
Figure Global Pre-Filled Heparin Lock Syringe Market Size and Growth Rate (2024-2033)
2 Pre-Filled Heparin Lock Syringe Market Growth Drivers and Constraints
The growth of the global pre-filled heparin lock syringe market is influenced by several key factors. Driving factors include:
Increasing Demand for Injectable Drugs: The rising prevalence of chronic diseases and the need for effective drug delivery methods have led to a surge in demand for pre-filled heparin lock syringes. These syringes offer a convenient and safe alternative to traditional vials, reducing the risk of contamination and medication errors.
Technological Advancements: Innovations in manufacturing processes and materials have improved the quality and reliability of pre-filled heparin lock syringes. Advances in sterilization techniques and packaging have further enhanced their safety and shelf life.
Regulatory Support: Governments and healthcare organizations are increasingly promoting the use of pre-filled syringes to minimize the risk of infections and improve patient outcomes. Regulatory bodies such as the FDA have reclassified pre-filled heparin lock syringes as medical devices, emphasizing their importance in healthcare settings.
Growing Homecare Market: The expansion of homecare services has led to an increased demand for pre-filled heparin lock syringes, as they offer a convenient solution for patients requiring long-term care.
However, there are also limiting factors that could hinder market growth:
High Initial Costs: The production of pre-filled heparin lock syringes involves advanced technology and strict quality control measures, leading to higher initial costs. This may limit their adoption in low-income regions.
Intense Competition: The market is highly competitive, with major players constantly vying for market share. This has led to price wars and reduced profit margins for manufacturers.
Regulatory Challenges: The stringent regulatory environment for medical devices can pose challenges for new entrants and existing players alike. Compliance with regulations can be costly and time-consuming, potentially slowing market expansion.
Economic Uncertainty: Economic downturns and fluctuations in healthcare spending can impact the demand for pre-filled heparin lock syringes. Budget constraints in public healthcare systems may lead to reduced investments in advanced medical devices..
3 Pre-Filled Heparin Lock Syringe Market Innovations and M&A Activities
The pre-filled heparin lock syringe market is characterized by continuous technological innovation and significant corporate activity. Technological innovations include:
Advanced Sterilization Techniques: Manufacturers are adopting advanced sterilization methods to ensure the highest level of safety and sterility in their products. These techniques help reduce the risk of contamination and improve overall product quality.
Improved Packaging: Innovations in packaging have led to the development of tamper-evident and easy-to-use designs. These features enhance patient safety and convenience, making pre-filled heparin lock syringes more attractive to healthcare providers.
Customizable Solutions: Companies are focusing on developing customizable syringes that can be tailored to meet specific clinical needs. This includes variations in syringe size, heparin concentration, and packaging options.
Corporate mergers and acquisitions are also shaping the market landscape:
Strategic Partnerships: Major players are forming strategic partnerships to expand their market reach and share resources. For example, Becton Dickinson and B. Braun have collaborated on various initiatives to enhance their product portfolios and improve market penetration.
Acquisitions: Companies are acquiring smaller, innovative startups to integrate new technologies and products into their existing offerings. This helps them stay competitive and adapt to changing market demands.
Market Expansion: Firms are investing in new manufacturing facilities and distribution networks to increase their global presence. This includes expanding into emerging markets with high growth potential.
In conclusion, the global pre-filled heparin lock syringe market is poised for significant growth, driven by increasing demand, technological advancements, and regulatory support. However, challenges such as high costs and intense competition must be navigated to ensure sustainable development. Companies that focus on innovation, strategic partnerships, and market expansion are likely to thrive in this dynamic industry..
4 Global Pre-Filled Heparin Lock Syringe Market Analysis by Type
In 2024, the global pre-filled heparin lock syringe market is forecasted to have a total value of 104.93 million USD. Among the different types, the 3ml syringe size is expected to hold a value of 9.30 million USD, accounting for approximately 8.86% of the total market value. The 5ml syringe size is projected to be the largest segment with a value of 79.81 million USD, representing about 76.06% of the total market value. Meanwhile, the 10ml syringe size is anticipated to reach 15.82 million USD, making up around 15.08% of the total market value. This distribution highlights the continued dominance of the 5ml syringe size, driven by its widespread application and versatility in various medical settings.
Table Global Pre-Filled Heparin Lock Syringe Market Size and Share by Type in 2024
Type | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
3ml Syringe Size | 9.30 | 8.86% |
5ml Syringe Size | 79.81 | 76.06% |
10ml Syringe Size | 15.82 | 15.08% |
5 Global Pre-Filled Heparin Lock Syringe Market Analysis by Application
In 2024, the global pre-filled heparin lock syringe market is projected to have a total volume of 126,348.5 K Units. Among the key applications, hospitals are expected to account for 96,720.2 K Units, representing approximately 76.56% of the total market volume. Outpatient clinics will contribute 8,970.0 K Units, which is about 7.10% of the total volume. Homecare settings are forecasted to have 4,025.2 K Units, or roughly 3.19% of the total. Meanwhile, pharmaceutical companies will account for 16,633.1 K Units, making up around 13.16% of the market volume. This distribution highlights the dominant role of hospitals in driving the demand for pre-filled heparin lock syringes, reflecting their extensive use in maintaining the patency of intravenous catheters and reducing the risk of blood clots in clinical settings.
Table Global Pre-Filled Heparin Lock Syringe Volume and Share by Application in 2024
Application | Volume in 2024 ((K Units)) | Market Share in 2024 (%) |
|---|---|---|
Hospitals | 96720.2 | 76.56% |
Outpatient Clinics | 8970.0 | 7.10% |
Homecare Settings | 4025.2 | 3.19% |
Pharmaceuticals Company | 16633.1 | 13.16% |
6 Global Pre-Filled Heparin Lock Syringe Market Analysis by Region
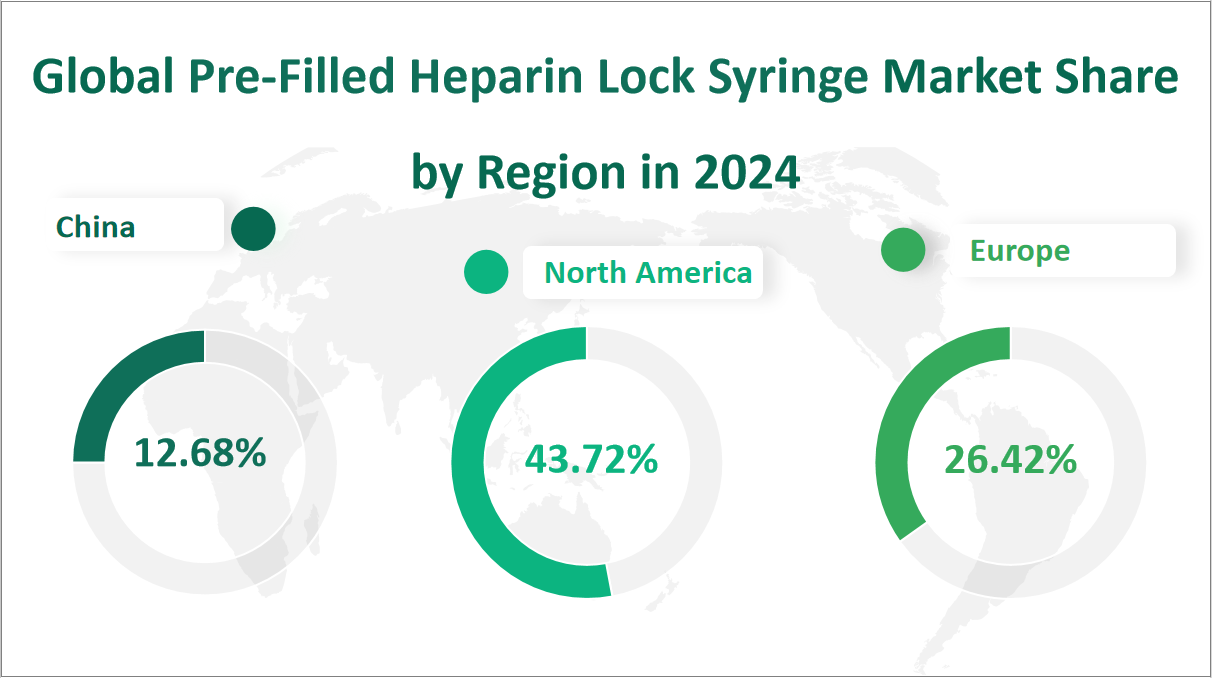
In 2024, the global pre-filled heparin lock syringe market is projected to see a total consumption of 126348.5 K Units. Among the key regions, North America is expected to lead with a consumption of 55245.4 K Units, accounting for approximately 43.72% of the global consumption. Europe follows with an estimated 33377.6 K Units, representing about 26.42% of the total. China is anticipated to consume 16021.1 K Units, making up around 12.68% of the global market. Japan is projected to have a consumption of 5281.8 K Units, or roughly 4.18% of the total. The Middle East and Africa are expected to consume 1895.9 K Units, representing approximately 1.50% of the global consumption. India is forecasted to have a consumption of 8894.5 K Units, or about 7.04% of the total. Lastly, South America is expected to consume 3.71.1 K Units, making up around 2.43% of the global market. This distribution highlights the significant contributions of North America and Europe, driven by their advanced healthcare systems and high demand for reliable medical devices, while emerging markets like China and India are also expected to play a substantial role in driving global consumption.
Figure Global Pre-Filled Heparin Lock Syringe Market Share by Region in 2024
7 Top 3 Companies of Global Pre-Filled Heparin Lock Syringe Market
7.1 Becton Dickinson
Company Introduction and Business Overview:
Becton Dickinson (BD) is a global medical technology company founded in 1897. Headquartered in Franklin Lakes, New Jersey, BD is renowned for developing, manufacturing, and selling medical devices, instrument systems, and reagents used by healthcare institutions, life science researchers, clinical laboratories, and the pharmaceutical industry. BD operates manufacturing facilities across America, Asia, and Europe, distributing its products worldwide. The company’s commitment to innovation and quality has solidified its position as a leader in the medical technology sector.
Products Offered:
BD offers a comprehensive range of pre-filled heparin lock syringes under the BD PosiFlush™ brand. These syringes are available in various sizes and concentrations, including 10 USP units per mL and 100 USP units per mL. Designed to maintain catheter patency, BD PosiFlush™ syringes are preservative-free, sterile, and compliant with the guidelines of the Infusion Nurses Society (INS). The product line is tailored to support different catheter maintenance practices, ensuring reliability and safety in clinical settings.
Sales Revenue in the Latest Year:
Becton Dickinson reported a production value of 28.29 million USD from its pre-filled heparin lock syringe segment. This figure reflects BD’s strong market position and its ability to meet the growing demand for high-quality medical devices. BD’s continuous investment in research and development, coupled with its extensive distribution network, has enabled it to maintain a significant market share and drive industry standards.
7.2 B. Braun
Company Introduction and Business Overview:
B. Braun Medical, Inc. is a leading manufacturer of medical products, founded in 1839. The company is headquartered in Bethlehem, Pennsylvania, and operates manufacturing facilities across Asia Pacific, Europe, and America. B. Braun offers a wide range of medical products, including admixture, dialysis, infusion therapy, duplex and premixed drugs, infusion pump systems, needle-free systems, and pain management products. The company’s commitment to innovation and quality has positioned it as a key player in the global healthcare industry.
Products Offered:
B. Braun’s pre-filled heparin lock syringe product line includes the Heparin 100 units per mL Pre-filled Flush Syringe. Available in a 5 mL size within a 10 mL syringe, these syringes are designed to reduce the risk of contamination and save preparation time. Key features include preservative-free formulation, latex-free materials, tamper-evident packaging, bar code labeling, and a two-year shelf life. B. Braun’s focus on safety and efficiency makes its products highly sought after in healthcare settings.
Sales Revenue in the Latest Year:
B. Braun reported a production value of 21.38 million USD from its pre-filled heparin lock syringe segment. This revenue underscores B. Braun’s strong market presence and its ability to deliver high-quality, reliable products. The company’s ongoing investment in technology and manufacturing processes has enabled it to maintain a competitive edge and meet the evolving needs of healthcare providers.
7.3 Excelsior Medical
Company Introduction and Business Overview:
Excelsior Medical, LLC is a leading manufacturer of pre-filled saline and heparin flush syringes, as well as syringe pump and pharmacy dispensing pump systems. Founded in 1989, Excelsior Medical is headquartered in Neptune, New Jersey. The company primarily serves the North American and European markets, focusing on delivering high-quality, innovative solutions for healthcare providers. Excelsior Medical’s commitment to quality and customer satisfaction has positioned it as a key player in the pre-filled syringe market.
Products Offered:
Excelsior Medical’s product portfolio includes the ZR® Saline Flush Syringes and Heparin Lock Syringes. These pre-filled syringes are terminally sterilized for additional safety and come in unit-dose packaging for ease of use. Key features include a two-year shelf life, barcoded labels for inventory control, latex and preservative-free materials, and compliance with USP 797 standards. Excelsior Medical’s products are designed to enhance patient care by reducing the risk of contamination and medication errors.
Sales Revenue in the Latest Year:
Excelsior Medical reported a production value of 12.57 million USD from its pre-filled heparin lock syringe segment. This revenue highlights the company’s strong market position and its ability to deliver innovative solutions to healthcare providers. Excelsior Medical’s focus on quality and customer service has enabled it to maintain a significant market share and drive industry standards.

