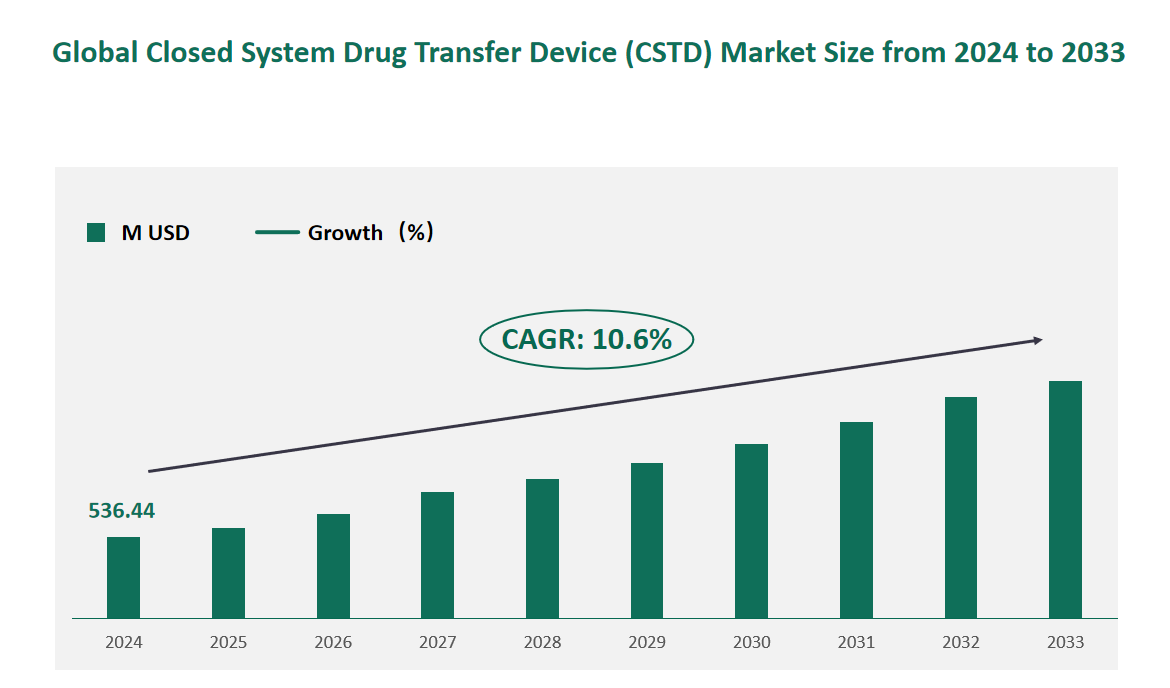
1 Global Closed System Drug Transfer Device (CSTD) Market Size (Value) and CAGR (2024-2033)
In 2024, the global Closed System Drug Transfer Device (CSTD) market was valued at USD 536.44 million, with a CAGR of 10.6% from 2024 to 2033.
Closed system drug-transfer device (CSTD) is a drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of hazardous drug or vapor concentrations outside the system.
Figure Global Closed System Drug Transfer Device (CSTD) Market Size (M USD) and CAGR 2024-2033
2 Closed System Drug Transfer Device (CSTD) Market Drivers
The Closed System Drug Transfer Device (CSTD) market is experiencing significant growth driven by various opportunities and favorable market dynamics. Two primary factors fueling this expansion are the increasing prevalence of cancer and the growing number of oncology drug approvals. Cancer is a global health concern, and its rising incidence has led to a higher demand for chemotherapy and other hazardous drug treatments. These drugs, while life-saving for patients, pose significant risks to healthcare workers if not handled properly. CSTDs play a crucial role in mitigating these risks by preventing the escape of harmful vapors and droplets during drug preparation and administration. The increasing number of oncology drugs approved by regulatory bodies further underscores the need for safer handling practices, thereby driving the adoption of CSTDs.
Another key driver is the support from related policies and regulatory guidelines. Governments and healthcare authorities worldwide have recognized the occupational hazards faced by healthcare workers when handling hazardous drugs. For instance, the United States Pharmacopeia (USP) <800> guidelines mandate the use of CSTDs when preparing and administering hazardous drugs, emphasizing the importance of protecting both patients and healthcare workers. These policies have significantly boosted the market by creating a regulatory framework that encourages the widespread adoption of CSTDs in healthcare settings.
3 Closed System Drug Transfer Device (CSTD) Market Challenges
Despite the significant opportunities driving the growth of the Closed System Drug Transfer Device (CSTD) market, several challenges hinder its widespread adoption and expansion. One of the primary challenges is the uncertainty regarding the impact of CSTDs on drug quality. Drug manufacturers are often unaware of the use of CSTDs with their products, especially in clinical trials. This lack of awareness can lead to potential issues with product quality and patient safety, as CSTDs might introduce exogenous particles or affect drug efficacy. Such uncertainties can negatively influence the perception of CSTDs and limit their adoption.
Another significant challenge is the limited awareness and education among healthcare professionals, particularly nurses and pharmacists, about the benefits and proper use of CSTDs. Many healthcare workers are unaware of the risks associated with handling hazardous drugs and may not fully understand the protective capabilities of CSTDs. This lack of awareness can result in low adoption rates, as healthcare organizations may not prioritize investing in these devices. Additionally, the high cost of implementing CSTDs poses a major obstacle. The financial burden of purchasing and maintaining CSTDs can be prohibitive for many healthcare facilities, especially those with limited budgets. The cost of implementation, combined with uncertainties about the long-term benefits, can deter organizations from adopting these devices.
4 Global Closed System Drug Transfer Device (CSTD) Market Size and Share by Type in 2024
Closed Vial Access Devices are designed to provide a secure and contamination-free connection between a drug vial and a syringe or an intravenous (IV) system. These devices eliminate the need for needles, reducing the risk of surface contamination and needlestick injuries. They also feature a mechanical barrier that traps vapors and prevents the escape of hazardous drug concentrations. In 2024, the market value for Closed Vial Access Devices is estimated at $178.44 million. This type is widely used in hospital settings, particularly in oncology and critical care units, where the handling of hazardous drugs is frequent.
Closed Syringe Safety Devices are engineered to enhance safety during the administration of hazardous drugs via syringes. These devices typically use a spring mechanism to extend a protective sheath over the needle after injection, thereby minimizing the risk of accidental needlestick injuries. They also ensure that the drug remains contained within the system, preventing the escape of harmful vapors. In 2024, the market value for Closed Syringe Safety Devices is projected to be $161.47 million. These devices are highly valued in clinical settings where precision and safety are paramount.
Closed Bag/Line Access Devices are designed to connect infusion bags to IV lines, ensuring a closed and leak-proof system for the transfer and administration of hazardous drugs. These devices prevent the ingress of environmental contaminants and the escape of drug vapors, thereby maintaining a sterile and safe environment. They are particularly useful in settings where continuous infusion of hazardous drugs is required. In 2024, the market value for Closed Bag/Line Access Devices is estimated at $196.54 million. Their versatility and ability to integrate seamlessly with existing IV systems make them a preferred choice in both hospital and clinic settings.
Table Global Closed System Drug Transfer Device (CSTD) Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
Closed Vial Access Devices | 178.44 | 33.26% |
Closed Syringe Safety Devices | 161.47 | 30.10% |
Closed Bag/Line Access Devices | 196.54 | 36.64% |
5 Global Closed System Drug Transfer Device (CSTD) Market Size and Share by Application in 2024
Hospitals represent the largest application segment for CSTDs, driven by their extensive use in oncology centers, critical care units, and other departments where hazardous drugs are frequently handled. Hospitals require robust and reliable CSTDs to protect healthcare workers from exposure to harmful substances, comply with regulatory standards, and ensure patient safety. In 2024, the market value for CSTDs in hospitals is estimated at $456.28 million. This segment accounts for approximately 85.06% of the total market share, reflecting the critical need for CSTDs in hospital settings.
Clinics, including outpatient facilities and specialized treatment centers, also benefit from the use of CSTDs, particularly in managing hazardous drugs for chemotherapy and other treatments. While the volume of hazardous drug handling in clinics may be lower compared to hospitals, the need for safety and regulatory compliance remains high. In 2024, the market value for CSTDs in clinics is projected to be $80.16 million, representing 14.94% of the total market share. The growth in this segment is driven by the increasing number of specialized clinics and the expansion of outpatient services.
Table Global Closed System Drug Transfer Device (CSTD) Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
Hospital | 456.28 | 85.05% |
Clinic | 80.16 | 14.95% |
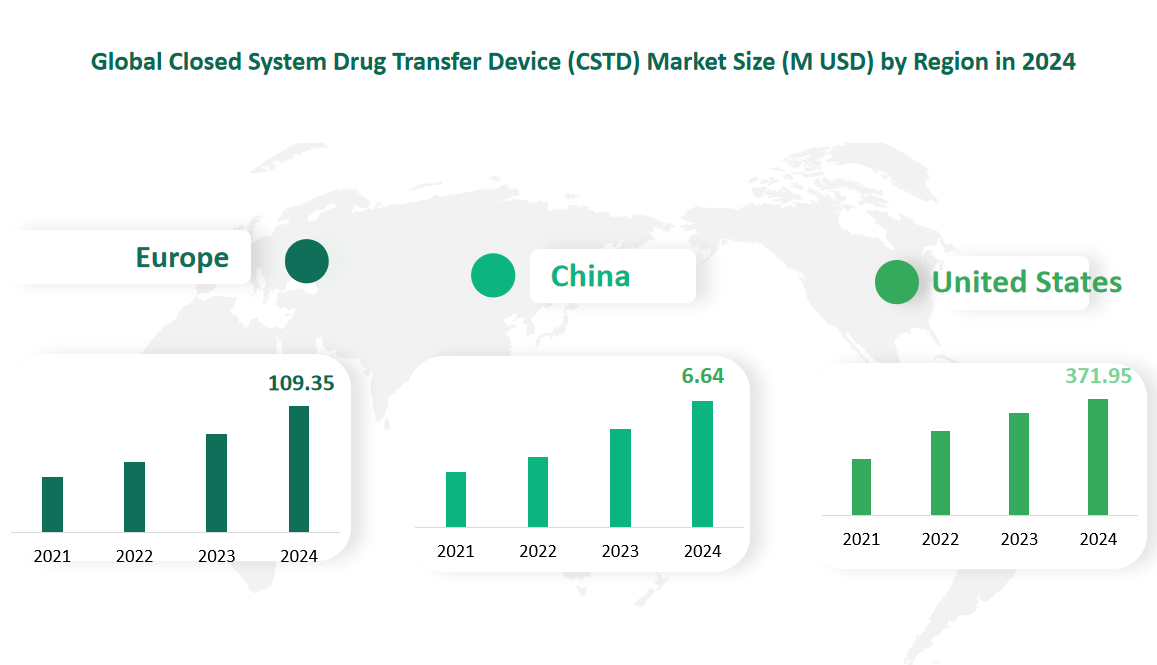
6 Global Closed System Drug Transfer Device (CSTD) Market Size by Region in 2024
United States is the largest regional market for CSTDs. The region’s strong healthcare infrastructure, high awareness of safety standards, and stringent regulatory requirements drive the widespread adoption of CSTDs. In 2024, the market value for CSTDs in United States is estimated at $371.95 million.
Europe is the second-largest regional market for CSTDs, driven by its robust healthcare systems and increasing focus on worker safety. European countries have adopted strict regulatory guidelines to protect healthcare workers from hazardous drug exposure, which has significantly boosted the market. In 2024, the market value for CSTDs in Europe is projected to be $109.35 million. Key markets within Europe include the United Kingdom, Germany, France, and Italy, where the adoption of CSTDs is particularly high.
Figure Global Closed System Drug Transfer Device (CSTD) Market Size by Region in 2024
7 Major Players in Global Closed System Drug Transfer Device (CSTD) Market
7.1 BD Medical
Company Profile: BD Medical, a subsidiary of Becton, Dickinson and Company, is a global medical technology company founded in 1897. It is headquartered in Franklin Lakes, New Jersey, USA, and operates worldwide, with manufacturing plants primarily located in North America, Asia, and Europe. BD Medical is renowned for its commitment to advancing healthcare through innovative solutions in medical research, diagnostics, and patient care.
Business Overview: BD Medical provides a wide range of medical devices and solutions aimed at improving healthcare safety and efficiency. Its product portfolio includes devices for medication management, infection prevention, and surgical procedures. BD Medical’s CSTD offerings are designed to enhance safety during the handling and administration of hazardous drugs, particularly in oncology settings.
Product Introduction: BD Medical’s CSTD product, the BD PhaSeal™ system, is a closed-system drug transfer device that prevents the ingress of environmental contaminants and the escape of hazardous drug vapors. It consists of three primary components: protector, injector, and connector, which together form a secure, closed system. The BD PhaSeal™ system is widely used in hospitals and clinics for the safe administration of chemotherapy medications.
Recent Financial Performance: In 2023, BD Medical’s CSTD revenue reached $245.72 million, with a gross margin of 53.25%.
7.2 Equashield
Company Profile: Equashield is a privately held medical device company founded in 2009. It is headquartered in the United States and primarily serves the North American market. Equashield is dedicated to providing innovative solutions for the safe handling of hazardous drugs, focusing on the protection of healthcare workers from exposure to harmful substances.
Business Overview: Equashield specializes in the development and manufacturing of closed-system transfer devices designed to meet the highest safety standards. Its products are widely recognized for their effectiveness in preventing the escape of hazardous drug vapors and droplets, thereby reducing the risk of occupational exposure. Equashield’s commitment to safety and innovation has positioned it as a leading provider of CSTDs globally.
Product Introduction: The EQUASHIELD® Closed System Transfer Device is an advanced CSTD designed to maintain airtight, leak-proof conditions during the transfer of hazardous drugs. It features a pressure equalization system that prevents the escape of vapors and aerosols, ensuring full protection for healthcare workers. The device is user-friendly and compatible with standard syringes and IV bags, making it a preferred choice in oncology and critical care settings.
Recent Financial Performance: In 2023, Equashield’s CSTD revenue was $94.82 million, with a gross margin of 52.13%.
7.3 ICU Medical
Company Profile: ICU Medical is a medical device company founded in 1984. It is headquartered in the United States and operates primarily in the Americas, Europe, the Middle East, and Africa. ICU Medical is known for its extensive portfolio of infusion therapy, oncology, and critical care products, designed to improve patient outcomes and healthcare worker safety.
Business Overview: ICU Medical specializes in the development of IV solutions, smart pumps, and needle-free connectors, with a focus on enhancing safety and efficiency in healthcare settings. Its CSTD offerings are designed to minimize exposure to hazardous drugs, ensuring compliance with regulatory standards and protecting healthcare workers from occupational hazards.
Product Introduction: ICU Medical’s CSTD products include ChemoLock™ and ChemoClave™, both of which feature intuitive designs and secure connections to prevent the ingress of contaminants and the escape of hazardous drug vapors. These devices are designed to be user-friendly and compatible with existing healthcare workflows, making them ideal for oncology and critical care applications.
Recent Financial Performance: In 2023, ICU Medical’s CSTD revenue reached $88.38 million, with a gross margin of 48.68%.

