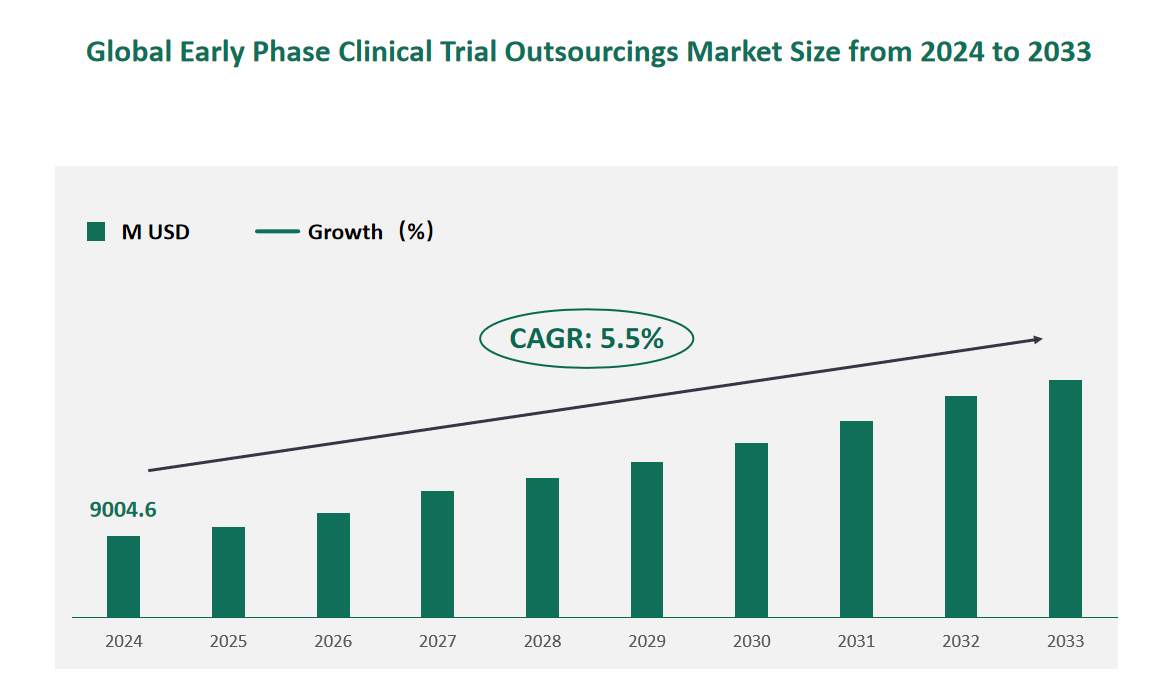
1 Global Early Phase Clinical Trial Outsourcings Market Size (Value) and CAGR (2024-2033)
In 2024, the global Early Phase Clinical Trial Outsourcings market was valued at USD 9004.6 million, with a CAGR of 5.5% from 2024 to 2033.
Early Phase Clinical Trial Outsourcing refers to the practice of contracting out the management and execution of early-stage clinical trials to specialized third-party service providers, known as Contract Research Organizations (CROs). These trials, typically classified as Phase 0, Phase 1, and Phase 2 studies, are crucial for assessing the safety, tolerability, and initial efficacy of new drugs or therapies in humans.
Figure Global Early Phase Clinical Trial Outsourcings Market Size (M USD) and CAGR 2024-2033
2 Early Phase Clinical Trial Outsourcings Market Drivers
Increasing Complexity of Drug Development: Modern drug development involves highly complex processes, requiring specialized expertise and advanced technologies. Contract Research Organizations (CROs) offer specialized services that can handle these complexities, making them an attractive option for pharmaceutical and biotechnology companies.
Cost and Time Efficiency: Outsourcing early-phase clinical trials can significantly reduce costs and accelerate the drug development timeline. CROs have the infrastructure and experience to manage trials more efficiently, allowing companies to bring products to market faster.
Regulatory Expertise: Navigating the regulatory landscape is a major challenge for drug developers. CROs specialize in regulatory compliance and can help companies meet the stringent requirements set by regulatory bodies, thereby reducing the risk of delays and failures.
Access to Global Patient Populations: CROs have extensive networks and can access diverse patient populations globally. This is particularly important for early-phase trials, which often require specific patient cohorts to assess the safety and efficacy of new drugs.
3 Early Phase Clinical Trial Outsourcings Market Restraints
Data Privacy and Security Concerns: Handling sensitive patient data requires strict adherence to data privacy regulations. Any breach or mishandling of data can lead to severe legal and financial consequences, which may deter some companies from outsourcing their trials.
Regulatory Hurdles: While CROs offer regulatory expertise, the constantly evolving regulatory environment can still pose challenges. Differences in regulations across countries can complicate the outsourcing process, requiring additional resources and time to ensure compliance.
Quality Control: Ensuring the quality and reliability of trial data is critical. Companies may face challenges in maintaining consistent quality standards across different CROs, especially when working with multiple service providers.
Communication and Coordination: Effective communication between the sponsor company and the CRO is essential for the success of a clinical trial. Miscommunication or lack of coordination can lead to delays, increased costs, and even trial failures.
4 Global Early Phase Clinical Trial Outsourcings Market Size and Share by Type in 2024
Regulatory Services: This segment is projected to reach $1,695.57 million in 2024. Regulatory Services are essential for ensuring that clinical trials comply with the stringent regulations set by health authorities. These services include obtaining regulatory approvals, preparing regulatory documents, and ensuring ongoing compliance throughout the trial.
Clinical Data Management (CDM): This segment is forecasted to be valued at $2,419.54 million in 2024. CDM involves the collection, management, and analysis of clinical trial data. Efficient data management is critical for the accuracy and reliability of trial results, making this segment a significant part of the outsourcing market.
Medical Writing: This segment is expected to reach $679.29 million in 2024. Medical Writing includes the preparation of clinical study reports, regulatory submissions, and other documentation required for clinical trials. Clear and accurate medical writing is essential for communicating trial results and meeting regulatory requirements.
Site Management: This segment is projected to be valued at $1,009.20 million in 2024. Site Management involves the coordination and management of clinical trial sites, ensuring that trials are conducted efficiently and effectively. This includes site selection, initiation, monitoring, and close-out activities.
Pharmacovigilance (PV): This segment is forecasted to reach $923.87 million in 2024. Pharmacovigilance involves the monitoring and reporting of adverse events and safety concerns during clinical trials. Ensuring patient safety is a critical aspect of clinical trials, making PV a vital component of the outsourcing market.
Table Global Early Phase Clinical Trial Outsourcings Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Marekt Share 2024 |
Regulatory Services | 1695.57 | 18.83% |
Clinical Data Management (CDM) | 2419.54 | 26.87% |
Medical Writing | 679.29 | 7.54% |
Site Management | 1009.20 | 11.21% |
Pharmacovigilance (PV) | 923.87 | 10.26% |
Risk-Based Monitoring | 864.50 | 9.60% |
Bio Statistical Services | 551.43 | 6.12% |
Protocol Development | 490.95 | 5.45% |
Others | 370.25 | 4.11% |
5 Global Early Phase Clinical Trial Outsourcings Market Size by Application in 2024
Pharmaceutical Companies: This segment is projected to reach $3,994.44 million in 2024. Pharmaceutical companies are the largest users of early-phase clinical trial outsourcing services, leveraging these services to accelerate drug development and reduce costs. These companies often outsource trials to gain access to specialized expertise and global patient populations.
Biopharmaceutical Companies: This segment is forecasted to be valued at $2,710.39 million in 2024. Biopharmaceutical companies, which focus on developing biologic drugs, also rely heavily on outsourcing to manage complex trials and navigate regulatory requirements. These companies often have limited in-house capabilities and benefit from the specialized services offered by CROs.
Drug Discovery Companies: This segment is expected to reach $1,387.61 million in 2024. Drug discovery companies, which are involved in the initial stages of drug development, use outsourcing services to streamline their research processes and access advanced technologies and expertise.
Medical Devices Companies: This segment is projected to be valued at $584.40 million in 2024. Medical devices companies outsource early-phase trials to test the safety and efficacy of new devices. These trials are critical for obtaining regulatory approvals and bringing new products to market.
Table Global Early Phase Clinical Trial Outsourcings Market Size by Application in 2024
Application | Market Size (M USD) 2024 |
Pharmaceutical Companies | 3994.44 |
Biopharmaceutical Companies | 2710.39 |
Drug Discovery Companies | 1387.61 |
Medical Devices Companies | 584.40 |
Other | 327.77 |
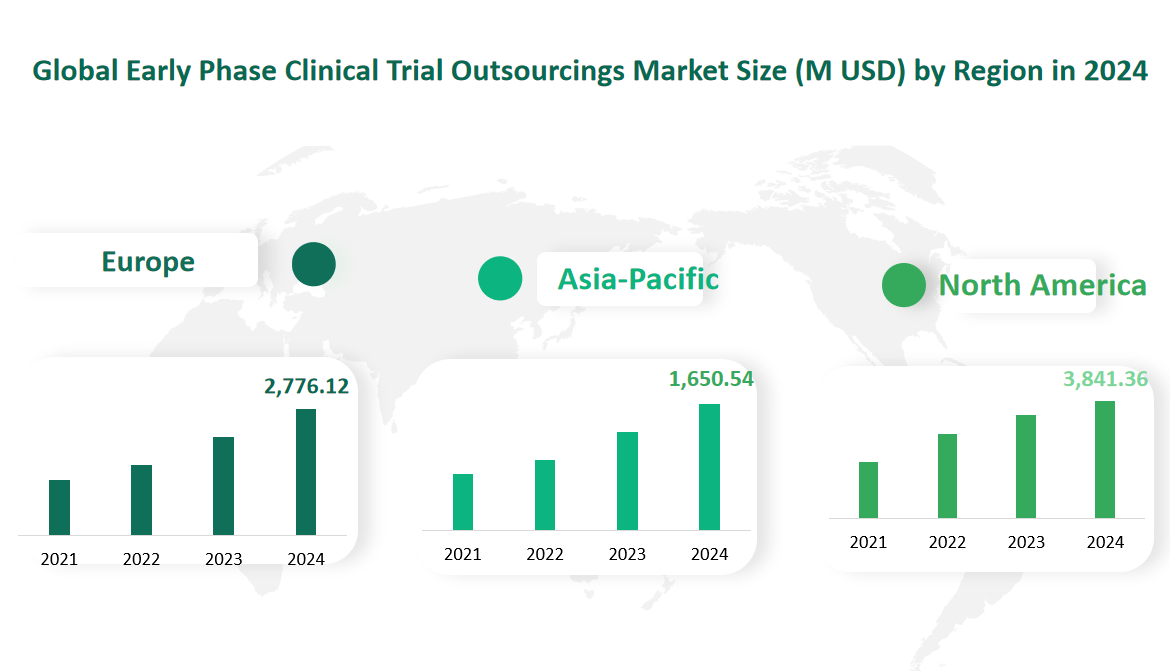
6 Global Early Phase Clinical Trial Outsourcings Market Size by Region in 2024
North America: This region is projected to reach $3,841.36 million in 2024. North America, led by the United States, is the largest market for early-phase clinical trial outsourcing due to its robust pharmaceutical industry and advanced healthcare infrastructure. The region is known for its high demand for specialized services and stringent regulatory requirements.
Europe: This region is forecasted to be valued at $2,776.12 million in 2024. Europe is a significant market, driven by its strong pharmaceutical industry and advanced healthcare systems. The region is also known for its stringent regulatory environment, which drives the demand for outsourcing services.
Asia-Pacific: This region is expected to reach $1,650.54 million in 2024. The Asia-Pacific region is the fastest-growing market, driven by the increasing number of clinical trials in countries like China and India. The region offers cost-effective services and access to a large patient population, making it an attractive destination for outsourcing.
Figure Global Early Phase Clinical Trial Outsourcings Market Size by Region in 2024
7 Major Players in Global Early Phase Clinical Trial Outsourcings Market
7.1 IQVIA
Company Profile: IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. Established in 1982, IQVIA operates worldwide and is headquartered in the United States. The company is known for its comprehensive suite of services, including regulatory services, clinical data management, medical writing, site management, pharmacovigilance, risk-based monitoring, bio-statistical services, and protocol development.
Business Overview: IQVIA’s business is segmented into three main areas: Commercial Solutions, Research & Development Solutions, and Integrated Engagement Services. The company’s Commercial Solutions segment offers cloud-based applications, real-world insights, and strategic consulting services. The Research & Development Solutions segment provides clinical trial management, data management, and biostatistical analysis. The Integrated Engagement Services segment focuses on enhancing client interactions and improving outcomes.
Products Analysis: IQVIA is recognized as an industry leader in Phase I trials, with a history of executing early clinical development trials globally. The company’s services are designed to enhance the speed, quality, and safety of Phase I studies, whether they involve first-in-human, first-in-patient, or healthy volunteer studies. IQVIA’s global resources and expertise make it a preferred partner for many pharmaceutical and biotechnology companies.
Recent Financial Performance: In the most recent year, IQVIA reported a revenue of $1,046.33 million from its Early Phase Clinical Trial Outsourcing services.
7.2 Syneos Health
Company Profile: Syneos Health is a leading integrated biopharmaceutical solutions company, offering a comprehensive suite of services across the entire spectrum of clinical development. Established in 1999, Syneos Health operates globally, with a strong presence in North America, Europe, the Middle East, Africa, the Asia-Pacific, and Latin America.
Business Overview: Syneos Health operates through two main segments: Clinical Solutions and Commercial Solutions. The Clinical Solutions segment provides a wide range of clinical development services, including full-service global studies, clinical monitoring, investigator recruitment, patient recruitment, data management, and study startup. The Commercial Solutions segment focuses on commercialization services, helping clients bring their products to market effectively.
Products Analysis: Syneos Health’s Phase I research services are designed to provide tactical options tailored to the early development of new drugs. The company’s multidisciplinary team of experts works closely with clients to guide them swiftly through the proof-of-concept phase, ensuring that initial human data are collected efficiently and effectively.
Recent Financial Performance: In the most recent year, Syneos Health reported a revenue of $872.10 million from its Early Phase Clinical Trial Outsourcing services.
7.3 Covance
Company Profile: Covance is a leading provider of early- and late-stage services to the pharmaceutical, biotechnology, and medical device industries. Established in 1987, Covance operates globally, offering a wide range of services that support the entire drug development process.
Business Overview: Covance’s early-stage development services include preclinical and clinical pharmacology services. The company’s late-stage services encompass biomarker services, full management of Phase II and III clinical studies, and lab testing services for various industries. Covance is known for its comprehensive and integrated approach to drug development, providing clients with a seamless transition from early to late-stage trials.
Products Analysis: Covance’s clinical pharmacology team brings over 30 years of direct drug development experience. The company partners with over 250 staff members fully dedicated to early patient studies and optimizes these studies with over 300 investigator sites globally. This extensive network and expertise make Covance a preferred choice for many clients seeking early-phase clinical trial services.
Recent Financial Performance: In the most recent year, Covance reported a revenue of $782.21 million from its Early Phase Clinical Trial Outsourcing services.

