1 Global Functional Service Providers Market Size (Revenue) and CAGR (2024-2033)
Global Functional Service Providers market generated revenue of USD 11460.66 Million in 2024 with a CAGR of 8.28% during 2024 to 2033.
Looking ahead, the market is poised for continued growth. The Compound Annual Growth Rate (CAGR) from 2024 to 2026 is projected to be 8.28%. This growth will be driven by several factors, including the increasing complexity of clinical research, advancements in technology, and the need for flexible and scalable solutions. Additionally, the ongoing impact of the COVID-19 pandemic has highlighted the importance of FSP models, as companies seek to navigate uncertainties and maintain operational efficiency.
In conclusion, the Functional Service Providers market in 2024 demonstrated strong growth and a dynamic landscape, with significant contributions from both established and emerging regions. As the industry continues to evolve, driven by technological advancements and increasing demand for specialized services, the market is expected to maintain its upward trajectory, reaching new heights by 2033.
Figure Global Functional Service Providers Market Size (M USD) Outlook (2024-2033)

2 Functional Service Providers Market Drivers
Table Drivers of Functional Service Providers
Drivers | Description |
Cost saving | FSP can reduce developer costs by reducing redundant activities. This can happen when project management is carried out in both pharmaceutical companies and contract research organization. These are expensive resources. If FSP partners use the systems and processes of pharmaceutical companies, supervision will also become simpler and cheaper. |
FSP can improve the flexibility of the company | The established full-service model, in which biopharmaceutical companies retain a preferred contract research organization (CRO) to perform the full range of clinical trial activities, has been increasingly complemented by a functional service provider (FSP) approach, which allows companies to engage discrete expert services—such as medical writing, biostatistics, or clinical monitoring—when and where they need them. Ability to move up and down in a given geographic area or treatment area plan according to the company’s priorities is important. Flexible resourcing boosts efficiency and accelerates timelines. |
Engagement | The FSP model has tended to last a long time, that’s because there’s a strong relationship between the two organizations and a clear definition of each organization’s responsibilities. The FSP model is an ongoing partnership with a shared commitment to overcome challenges—such as slow recruitment or flaws in the protocol—collaboratively, leading to less finger-pointing. |
3 Global Functional Service Providers Market by Type in 2024
The Functional Service Providers (FSP) market is characterized by a diverse range of service types, each tailored to meet specific needs within the pharmaceutical, biotech, and medical device industries. In 2024, the market was segmented into several key product types, including Regulatory and Monitoring, Data and Project Management, Biostatistics, Statistical Programming, and Others.
Regulatory and Monitoring services are critical for ensuring compliance with regulatory standards and maintaining oversight throughout the drug development process. In 2024, this segment had a market value of $2,338.53 million USD and accounted for approximately 20.40% of the total FSP market. These services are essential for managing quality, progress, and financial aspects of clinical trials, thereby expanding internal supervision capabilities without requiring additional full-time employees (FTEs).
Data and Project Management emerged as the largest segment within the FSP market in 2024, with a market value of $2,958.06 million USD and a share of 25.81%. This segment encompasses database settings, data review methods, and project management solutions that optimize trial processes and timelines. The demand for efficient data management and project oversight has driven the growth of this segment, making it a cornerstone of the FSP industry.
Biostatistics services provide essential support for trial design, statistical analysis plans, and regulatory submissions. In 2024, the Biostatistics segment had a market value of $1,992.78 million USD and accounted for 17.39% of the total market. The expertise of biostatisticians is crucial for ensuring the scientific validity and regulatory compliance of clinical trials, thereby contributing significantly to the overall success of drug development programs.
Statistical Programming involves the transformation of clinical trial data into comprehensive analyses, supporting decision-making and regulatory submissions. This segment had a market value of $1,475.95 million USD in 2024, representing 12.88% of the total FSP market. The demand for skilled statistical programmers has grown as the complexity of clinical trials increases, driving the need for robust data analysis and reporting solutions.
Among these product types, Data and Project Management had the largest market share in 2024, driven by the increasing complexity of clinical trials and the need for efficient project oversight. However, the fastest-growing segment was Regulatory and Monitoring, which experienced a significant increase in demand due to heightened regulatory scrutiny and the need for robust compliance frameworks.
Table Global Functional Service Providers Market Size and Share by Type in 2024
Type | Market Size (M USD) | Market Share (%) |
|---|---|---|
Regulatory and Monitoring | 2,338.53 | 20.40% |
Data and Project Management | 2,958.06 | 25.81% |
Biostatistics | 1,992.78 | 17.39% |
Statistical Programming | 1,475.95 | 12.88% |
Others | 2,695.33 | 23.52% |
Total | 11,460.66 | 100.00% |
4 Global Functional Service Providers Market by Application in 2024
The Functional Service Providers market serves a wide range of applications, primarily focused on supporting the pharmaceutical, biotech, and medical device industries. In 2024, the market was segmented into three main applications: Pharma and Biotech Companies, Medical Device Companies, and Others.
Pharma and Biotech Companies are the primary users of FSP services, leveraging these solutions to enhance efficiency, reduce costs, and accelerate drug development. In 2024, this segment had a market value of $9,709.70 million USD and accounted for 84.73% of the total FSP market. The adoption of FSP models has allowed these companies to access scalable, expert-level resources without the burden of maintaining large in-house teams. This has led to significant cost savings and improved operational flexibility, making it the largest application area within the FSP market.
Medical Device Companies also benefit from FSP services, particularly in areas such as clinical monitoring, data management, and regulatory compliance. In 2024, this segment had a market value of $1,539.11 million USD and accounted for 13.43% of the total market. The growth of this segment is driven by the increasing complexity of medical device trials and the need for specialized expertise in regulatory and clinical processes.
Among these applications, Pharma and Biotech Companies had the largest market share in 2024, driven by their extensive use of FSP models to optimize clinical trial processes and reduce development costs. This segment is expected to continue dominating the FSP market due to the ongoing demand for efficient and flexible solutions in drug development. The fastest-growing application area was Medical Device Companies, which experienced a significant increase in demand due to the growing complexity of medical device trials and the need for specialized regulatory support.
Table Global Functional Service Providers Market Size and Share by Application in 2024
Application | Market Size (M USD) | Market Share (%) |
|---|---|---|
Pharma and Biotech Companies | 9,709.70 | 84.73% |
Medical Device Companies | 1,539.11 | 13.43% |
Others | 211.85 | 1.84% |
Total | 11,460.66 | 100.00% |
5 Global Functional Service Providers Market by Region in 2024
The Functional Service Providers (FSP) market is a global industry that plays a crucial role in supporting the pharmaceutical, biotech, and medical device sectors. In 2024, the market exhibited significant regional variations in terms of size and growth, reflecting the diverse economic, regulatory, and technological environments across the world.
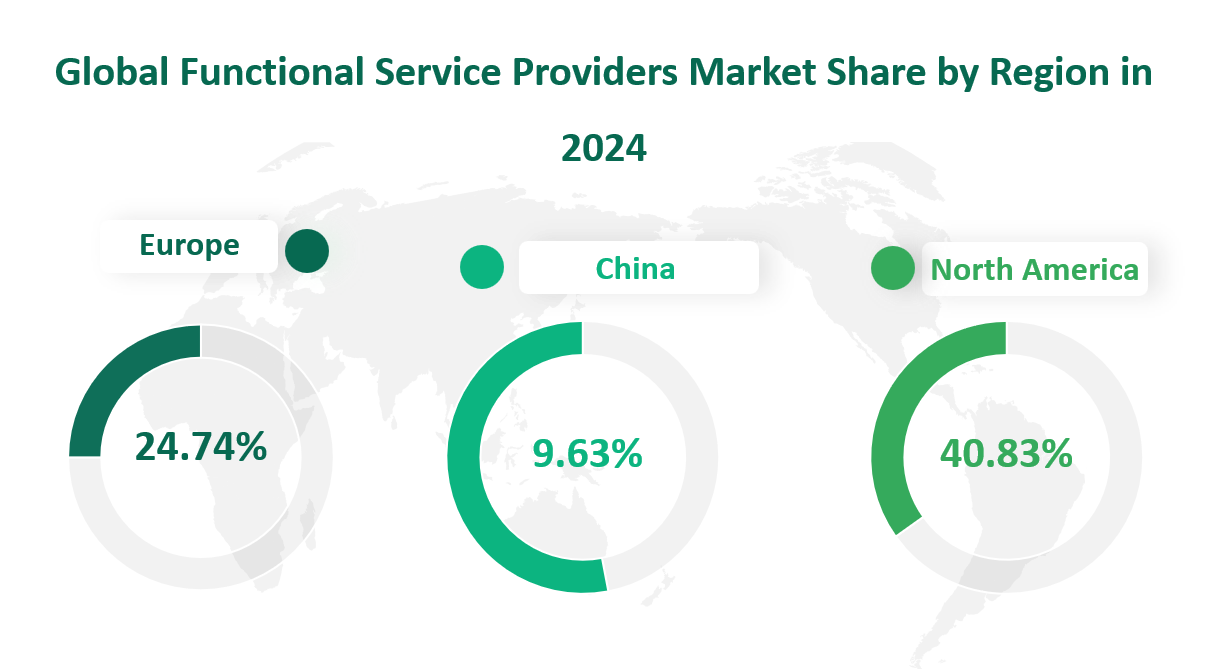
North America emerged as the largest regional market, accounting for 40.83% of the global FSP market revenue. This dominant position is attributed to the region’s advanced healthcare infrastructure, robust regulatory frameworks, and a high concentration of major pharmaceutical and biotech companies. The United States, in particular, has been a key driver of this market, with its well-established clinical research ecosystem and significant investments in drug development and innovation. In 2024, North America’s FSP market value reached $4,679.58 million USD, reflecting its central role in the industry.
Europe, known for its strong pharmaceutical industry and stringent regulatory environment, held the second-largest market share at 24.74%. The European market value in 2024 was $2835.07 million USD. The region’s growth is driven by its sophisticated healthcare systems, extensive research and development activities, and a growing emphasis on clinical trial efficiency. Countries such as the United Kingdom, Germany, and France have been particularly active in adopting FSP models to optimize their clinical trial processes and reduce operational costs.
China, one of the fastest-growing economies globally, has also become a significant player in the FSP market. In 2024, China’s market share reached 9.63%, with a market value of $1103.36 million USD. The rapid growth of China’s pharmaceutical and biotech industries, coupled with increasing government support for innovation and healthcare development, has driven the adoption of FSP models. Chinese companies are leveraging these services to enhance their clinical trial capabilities and meet regulatory requirements more efficiently.
Japan, with its highly developed healthcare and pharmaceutical sectors, accounted for 4.82% of the global FSP market in 2024, valued at $552.01 million USD. Japan’s market is characterized by a focus on high-quality clinical research and regulatory compliance. Despite its relatively smaller market share compared to North America and Europe, Japan remains a critical region due to its advanced technological infrastructure and strong emphasis on innovation.
The Middle East and Africa region, while smaller in market size, showed notable growth potential. In 2024, it accounted for 3.20% of the global market, valued at $366.38 million USD. The region’s growth is driven by increasing healthcare investments, improving regulatory frameworks, and a growing demand for outsourced clinical research services.
India, another emerging market, held a 5.51% share in 2024, with a market value of $631.31 million USD. India’s vast talent pool, cost-effective service offerings, and growing biotech sector have positioned it as an attractive destination for FSP services. The country’s market is expected to grow rapidly as more global companies seek to leverage its competitive advantages.
South America, with a market share of 4.25% in 2024, valued at $487.12 million USD, is also emerging as a significant player. The region’s diverse economy and growing healthcare sector are driving the adoption of FSP models, particularly in countries like Brazil and Argentina.
Among these regions, North America was the largest market by revenue in 2024, driven by its advanced healthcare infrastructure and significant investments in drug development. However, the fastest-growing region was China, where rapid economic development, increasing government support for healthcare innovation, and the growing biotech sector have fueled significant expansion in the FSP market.
Table Global Functional Service Providers Market Size, Region Wise in 2024
Region | Market Size (M USD) | Market Share (%) |
|---|---|---|
North America | 4679.58 | 40.83% |
Europe | 2835.07 | 24.74% |
China | 1103.36 | 9.63% |
Japan | 552.01 | 4.82% |
Middle East and Africa | 366.38 | 3.20% |
India | 631.63 | 5.51% |
South America | 487.12 | 4.25% |
Total | 11,460.66 | 100.00% |
Figure Global Functional Service Providers Market Share, Region Wise in 2024
6 Global Functional Service Providers Market Top 3 Players
Company Introduction and Business Overview
IQVIA is a global leader in healthcare research and analytics, providing a wide range of services to the pharmaceutical, biotech, and medical device industries. Established in 1982, IQVIA has a global presence and is renowned for its innovative solutions in clinical research, data analytics, and technology. The company’s mission is to leverage data and technology to drive healthcare forward and improve human health.
Products and Services
IQVIA offers a comprehensive suite of Functional Service Provider (FSP) solutions, including regulatory and monitoring services, data and project management, biostatistics, and statistical programming. Its FSP model is designed to provide flexibility, scalability, and cost-effectiveness, allowing clients to optimize their clinical trial processes and accelerate drug development.
Revenue in 2021
In 2021, IQVIA’s revenue from FSP services reached $1,451.90 million USD, solidifying its position as a dominant player in the market. The company’s strong performance is attributed to its extensive portfolio of services, advanced analytics capabilities, and strategic partnerships with leading pharmaceutical companies.
Company Introduction and Business Overview
Covance, a subsidiary of LabCorp, is a leading global life sciences company specializing in contract research services. Established in 1996, Covance provides comprehensive solutions for drug development, including clinical trials, laboratory services, and data management. The company is known for its innovative approach and commitment to advancing health through scientific excellence.
Products and Services
Covance’s FSP model, known as FSPx, offers customized solutions in clinical analytics and operations. Its services include data management, biostatistics, statistical programming, and e-clinical solutions. Covance’s FSPx model is designed to provide flexibility and scalability, allowing clients to optimize their clinical trial processes and reduce operational costs.
Revenue in 2021
In 2021, Covance’s revenue from FSP services was $1,120.72 million USD. The company’s growth is driven by its extensive experience in clinical research, strong regulatory compliance, and innovative solutions that meet the evolving needs of the pharmaceutical and biotech industries.
Company Introduction and Business Overview
Parexel International Corporation is a global biopharmaceutical services company that provides contract research, consulting, medical communications, and technology solutions. Established in 1982, Parexel has a global presence and is known for its expertise in clinical trial management and regulatory compliance. The company’s mission is to accelerate the development of life-saving and life-improving drugs through innovative solutions.
Products and Services
Parexel’s FSP model offers a range of services, including regulatory and monitoring, data and project management, biostatistics, and statistical programming. The company’s FSP solutions are designed to provide flexibility and scalability, allowing clients to optimize their clinical trial processes and improve operational efficiency.
Revenue in 2021
In 2021, Parexel’s revenue from FSP services reached $917.30 million USD. The company’s strong performance is attributed to its comprehensive portfolio of services, innovative solutions, and strategic partnerships with leading pharmaceutical and biotech companies.
Table Global Functional Service Providers Revenue Share of Top3 Players in 2021
Company | 2021 |
IQVIA | 16.29% |
Covance | 12.57% |
Parexel | 10.29% |

