1 Global Glioblastoma Multiforme Treatment Market Insight Analysis
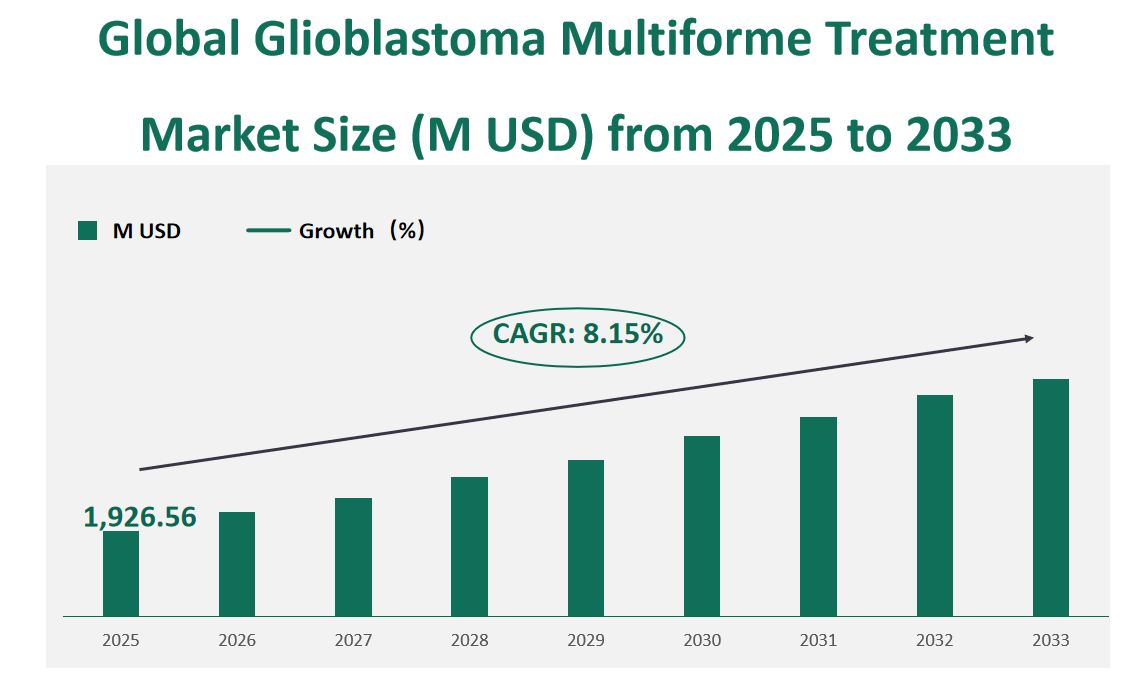
The global glioblastoma multiforme treatment market is expected to be valued at USD 1,926.56 million in 2025, with a CAGR of 8.15% from 2025 to 2033.
Glioblastoma Multiforme is a fast-growing and aggressive brain tumor. It invades nearby brain tissue but generally does not spread to distant organs. Glioblastoma Multiforme Treatment can slow down the growth of tumors and alleviate symptoms. Different types of treatment drugs, it is divided into Bevacizumab, Temozolomide, and Carmustine.
Figure Global Glioblastoma Multiforme Treatment Market Size (M USD) and CAGR (2025-2033)
2 Glioblastoma Multiforme Treatment Market Growth Drivers and Restraints
Population aging and rising prevalence
The global population is aging significantly, with the proportion of people over 60 years old increasing from 1 billion in 2020 to 2.1 billion in 2050. The elderly population has a high prevalence of GBM (accounting for 80% of malignant brain tumors) due to decreased immunity and accumulated gene mutations. According to data from the World Health Organization, there were 19.3 million new cancer cases worldwide in 2020, of which GBM cases continued to increase with aging, directly driving the demand for treatment.
Policy support and improved medical accessibility
Expanded medical insurance coverage: US Medicare Part D includes GBM treatment drugs (such as bevacizumab) in reimbursement, reducing the burden on patients; China has accelerated the listing of innovative drugs through the “priority approval of anticancer drugs” policy (such as the 2019 “Notice on Accelerating the Listing of Anticancer Drugs”).
Policy tilt in emerging markets: China’s “14th Five-Year Plan” clearly supports the biopharmaceutical industry, focuses on the development of malignant tumor treatment drugs, and promotes local companies’ R&D investment.
Technological progress and breakthroughs in new drug development
The rise of immunotherapy: immune checkpoint inhibitors (such as PD-1/PD-L1 monoclonal antibodies) and CAR-T cell therapy (targeting EGFRvIII, IL-13Rα2) have entered clinical trials, providing new options for recurrent GBM.
Gene and epigenetic analysis: Optimize chemotherapy regimens (such as temozolomide combined with radiotherapy) through precise typing to prolong patient survival.
Drug delivery technology innovation: Nanotechnology breaks through the blood-brain barrier (BBB) restrictions and increases the concentration of targeted drugs (such as liposome-encapsulated chemotherapy drugs).
Market demand and consumption upgrade
Global medical expenditures have increased, and patients are more willing to pay for highly effective therapies. For example, the size of the US GBM treatment market has dropped from US$10.13 billion in 2018 to US$8.07 billion in 2022, but is expected to rebound to US$11.86 billion in 2028, with a compound annual growth rate (CAGR) of 5.37%, reflecting the market potential of innovative therapies.
High R&D cost and long cycle
The average time for new drug development is 10-15 years and the cost exceeds $1 billion. For example, Merck’s Temozolomide (Temodar) needs to be verified by Phase III clinical trials, and GBM heterogeneity leads to a high failure rate of clinical trials (for example, a CAR-T therapy in 2022 terminated Phase III trials due to insufficient efficacy). High risks discourage small and medium-sized enterprises, and the market is concentrated in giants such as Roche and Amgen.
Blood-brain barrier and drug resistance challenges
The BBB blocks 98% of small molecule drugs and 100% of large molecule drugs from entering the brain, limiting the therapeutic effect. In addition, GBM cells are prone to chemotherapy resistance (for example, about 50% of MGMT methylation-positive patients are resistant to temozolomide), requiring combination therapy but with superimposed toxicity.
Uncertainty in policies and regulations
Price control: The European Medicines Agency (EMA) implements strict pricing reviews on innovative drugs, limiting corporate profit margins.
Regulatory barriers: Generic drug approval requires bioequivalence testing, which takes 2-3 years, delaying the launch of low-priced alternatives (e.g., generic temozolomide produced by Indian pharmaceutical companies must be approved by the FDA before entering the US market).
Economic and geopolitical impact
Supply chain disruption: The conflict between Russia and Ukraine has damaged medical facilities in Eastern Europe, affecting clinical trials and drug supply (e.g., a GBM research center in Ukraine was forced to close, delaying data collection).
Regional market imbalance: Due to the lack of medical resources in low-income countries (such as Africa), the five-year survival rate of GBM patients is less than 5%, and market demand is difficult to release.
3 Technological Innovations in the Glioblastoma Multiforme Treatment Market
Immunity and gene therapy
CAR-T cell therapy: Amgen’s AMG 102 targets IL-13Rα2, with an objective response rate (ORR) of 23% in patients with recurrent GBM; Novartis’ CT053 targets B-cell maturation antigen (BCMA) and enters Phase I to explore the synergistic effect of combined radiotherapy.
Oncolytic virus therapy: Merck’s ONCOS-102 (herpes simplex virus derivative) activates immune response through intratumoral injection, and Phase II trials show that the median survival is extended to 12.5 months.
Precision medicine and combination therapy
Molecular typing guides treatment: Prognosis is distinguished by IDH mutation status. IDH wild-type patients are given priority to bevacizumab, and mutant patients try isocitrate dehydrogenase inhibitors (such as ivosidenib).
Multimodal combination: Surgery + radiotherapy + electric field therapy (such as Novocure’s Tumor Treating Fields) extends the median survival from 15 months to 20.9 months, and was approved by the FDA for newly diagnosed GBM in 2023.
Drug delivery and dosage form innovation
Implantable sustained-release technology: Eisai’s GLIADEL® wafer (carmustine sustained-release implant) is locally administered after surgery to maintain high concentrations of chemotherapy drugs and reduce systemic toxicity. In 2022, it was approved in Japan to expand its indications to newly diagnosed GBM.
Nano-targeted system: Pfizer’s ZIRABEV (bevacizumab biosimilar) combines pegylated liposomes to extend the circulation half-life and reduce the frequency of administration.
Head companies expand pipelines
Roche acquires Good Therapeutics (2022): Acquires the PD-1-regulated IL-2 agonist platform to develop GBM immune combination therapy. The transaction amount was not disclosed, but it is expected to enhance its competitiveness in the field of immune checkpoints.
Amgen acquires Horizon Therapeutics (2022, $27.8 billion): Incorporates anti-IL-17A monoclonal antibody tezepelumab to explore its role in regulating GBM microenvironment and supplement its own layout in the intersection of inflammation and tumors.
Competition between biosimilars and generics
Sandoz launches temozolomide generics: Enters the European market in 2023 at a price 30% lower than the original drug, seizes Merck’s Temodar share, and drives market prices down.
Pfizer and Amgen collaborate to develop bevacizumab biosimilars: ZIRABEV was approved in 2021, with sales of $1.89 billion in 2023, squeezing Roche’s Avastin market space (Roche’s share fell from 80.8% in 2018 to 48.2% in 2023).
Adjustment of regional market layout
Takeda Pharmaceutical expands GLIADEL® wafer indications in Japan: in 2023, it will be added for newly diagnosed GBM in combination with radiotherapy, and increase revenue by leveraging local market advantages (the Japanese GBM treatment market size will be US$1.31 billion in 2023, with a CAGR of 5.31%).
India’s Dr. Reddy’s Laboratory launches carmustine generics: penetrating the Southeast Asian market at a low price, with a market share of 18% in India in 2023, challenging the position of original drug companies such as Merck.
4 Global Glioblastoma Multiforme Treatment Market Size by Type
Bevacizumab is the dominant type in the glioblastoma multiforme treatment market. In 2025, it is expected to generate a revenue of $1,561.15 million, accounting for approximately 81.03% of the total market share. Bevacizumab, marketed under the brand name Avastin, is a monoclonal antibody that targets vascular endothelial growth factor (VEGF). It is widely used to treat various types of cancers, including glioblastoma multiforme. Its effectiveness in inhibiting tumor growth and its broad approval in over 130 countries contribute to its significant market share. The growth of Bevacizumab is further supported by ongoing research and development efforts aimed at expanding its applications and improving its efficacy.
Temozolomide is another key player in the market, with a projected revenue of $271.22 million in 2025. It holds a market share of 14.08% in the same year. Temozolomide is an oral alkylating agent primarily used for the treatment of newly diagnosed glioblastoma multiforme and refractory anaplastic astrocytoma. Its ease of administration and proven efficacy in extending patient survival make it a preferred choice for many healthcare providers. The market growth of Temozolomide is also driven by its inclusion in standard treatment protocols for brain tumors and ongoing clinical trials exploring its use in combination with other therapies.
Carmustine, also known as BCNU, is a chemotherapy drug used for treating brain tumors, non-Hodgkin’s lymphoma, and Hodgkin’s lymphoma. In 2025, it is expected to generate a revenue of $94.19 million, representing a market share of 4.89%. Carmustine is particularly effective in treating glioblastoma multiforme due to its ability to cross the blood-brain barrier. Its market share is influenced by its use in combination therapies and its role in treating recurrent and refractory cases of brain tumors. Despite its smaller market share compared to Bevacizumab and Temozolomide, Carmustine remains a critical component in the treatment arsenal for glioblastoma multiforme.
Table Global Glioblastoma Multiforme Treatment Market Size and Share by Type in 2025
|
Type |
Market Size (M USD) 2025 |
Market Share 2025 |
|---|---|---|
|
Bevacizumab |
1561.15 |
81.03% |
|
Temozolomide |
271.22 |
14.08% |
|
Carmustine |
94.19 |
4.89% |
5 Global Glioblastoma Multiforme Treatment Market Size by Application
Hospitals play a dominant role in the GBM treatment market. In 2025, the market revenue for GBM treatment in hospitals is projected to be 1303.03 million USD. This accounts for a significant market share of 67.64%. Hospitals are the primary providers of comprehensive GBM treatment, which often involves complex procedures such as neurosurgical operations, high – tech radiotherapy, and multi – drug chemotherapy. They are equipped with advanced medical facilities and a team of specialized medical professionals, including neurosurgeons, oncologists, and radiologists. The high revenue in hospitals can also be attributed to the fact that they are more likely to participate in clinical trials and offer the latest and most expensive treatment options, such as immunotherapy and targeted drug therapies.
Clinics are the second – largest segment in the GBM treatment market. In 2025, the market revenue for GBM treatment in clinics is expected to be 506.61 million USD, holding a market share of 26.30%. Clinics typically provide follow – up care, outpatient chemotherapy, and some basic diagnostic services for GBM patients. They are more accessible and convenient for patients who do not require inpatient care or complex surgical procedures. Some clinics also collaborate with hospitals to offer a continuum of care, which helps in sharing the load of patient management and contributes to the overall market revenue.
Table Global Glioblastoma Multiforme Treatment Market Size and Share by Application in 2025
|
Application |
Market Size (M USD) 2025 |
Market Share 2025 |
|---|---|---|
|
Hospitals |
1303.03 |
67.64% |
|
Clinics |
506.61 |
26.30% |
|
Others |
116.92 |
6.07% |
6 Global Glioblastoma Multiforme Treatment Market Size by Region
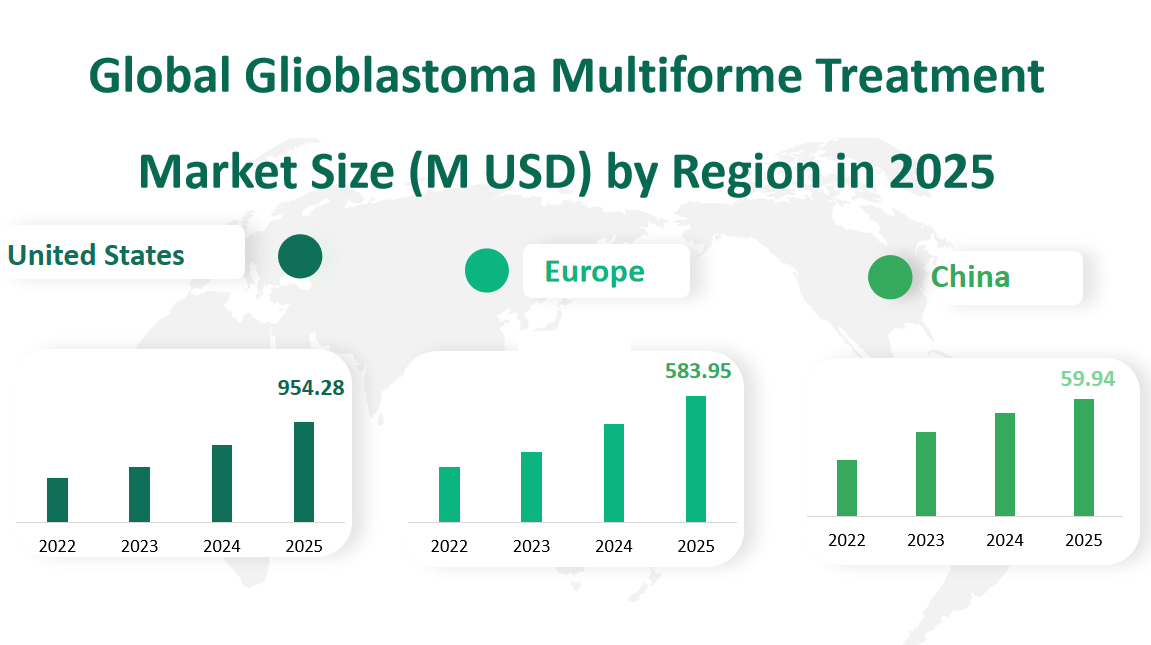
The United States is projected to be the largest market for glioblastoma multiforme treatment in 2025, with a market size of $954.28 million. This significant market share can be attributed to several factors. The U.S. has a well-established healthcare infrastructure, a high incidence of brain tumors, and strong government support for medical research and development. Additionally, the presence of major pharmaceutical companies and advanced research facilities contributes to the development of innovative treatments. The market in the U.S. is expected to grow steadily due to increasing healthcare expenditure, rising geriatric population, and continuous advancements in treatment options. The growth is also driven by the availability of novel therapies and the expansion of insurance coverage for these treatments.
Europe is anticipated to hold a substantial share of the market in 2025, with a projected market size of $583.95 million. The European market is characterized by a mix of developed healthcare systems across various countries, leading to a diverse demand for glioblastoma multiforme treatments. Factors contributing to the market growth include an aging population, increased awareness about brain tumors, and the presence of significant pharmaceutical companies. Additionally, the European Union’s efforts to harmonize healthcare policies across member states are expected to facilitate better access to treatments. The market in Europe is also influenced by the ongoing research collaborations and the development of new drugs, which are expected to drive demand further.
China is projected to have a market size of $59.94 million for glioblastoma multiforme treatment in 2025. The market in China is growing rapidly due to several factors, including a large population base, increasing healthcare expenditure, and government initiatives to improve healthcare access and quality. The rising prevalence of brain tumors and the growing awareness about the importance of early diagnosis and treatment are also contributing to the market growth. China’s market is also influenced by the increasing focus on domestic research and development, with local pharmaceutical companies investing in the development of new treatments. Furthermore, partnerships with international pharmaceutical companies are expected to introduce more advanced therapies into the market, further driving growth.
Figure Global Glioblastoma Multiforme Treatment Market Size (M USD) by Region in 2025
7 Global Glioblastoma Multiforme Treatment Market Analysis by Major Players
F. Hoffmann-La Roche
Company Profile
F. Hoffmann-La Roche, commonly known as Roche, is a global leader in the pharmaceutical and diagnostics industries. Founded in 1896 in Basel, Switzerland, Roche has a long history of innovation and excellence in healthcare. The company is renowned for its commitment to research and development, aiming to improve the lives of patients through groundbreaking therapies and diagnostic solutions. Roche operates globally, with a significant presence in North America, Europe, and Asia-Pacific regions.
Business Overview
Roche’s business encompasses a wide range of therapeutic areas, including oncology, immunology, infectious diseases, and neuroscience. The company is particularly strong in oncology, where it offers a diverse portfolio of products aimed at treating various types of cancer. Roche’s business strategy focuses on integrating pharmaceuticals and diagnostics to create personalized healthcare solutions. This approach allows the company to develop targeted therapies that improve patient outcomes and reduce healthcare costs.
Product Offered
Roche’s flagship product in the glioblastoma multiforme treatment market is Avastin (Bevacizumab). Avastin is a monoclonal antibody that inhibits the growth of blood vessels in tumors, thereby slowing their growth and spread. It is approved for the treatment of multiple cancers, including glioblastoma multiforme, and is used in combination with other therapies to enhance efficacy. Roche’s continuous investment in research has led to the development of additional products and therapies aimed at improving the treatment landscape for brain tumors.
Amgen Inc.
Company Profile
Amgen Inc., founded in 1980, is a leading biotechnology company headquartered in Thousand Oaks, California. Amgen is known for its innovative approach to developing and commercializing medicines that address significant unmet medical needs. The company has a strong focus on biotechnology and has made significant contributions to the field of oncology, cardiovascular diseases, and neuroscience. Amgen’s commitment to scientific excellence and patient care has positioned it as a key player in the global healthcare industry.
Business Overview
Amgen’s business strategy centers on leveraging advanced biotechnology to develop therapies that improve patients’ lives. The company operates globally, with a strong presence in North America, Europe, and Asia. Amgen’s portfolio includes a range of products targeting various diseases, with a particular emphasis on oncology and immunology. The company’s business model focuses on innovation, strategic partnerships, and continuous research to stay at the forefront of biotechnology advancements.
Product Offered
Amgen’s contribution to the glioblastoma multiforme treatment market includes MVASI™ (Bevacizumab-awwb), a biosimilar to Avastin. MVASI™ is approved for the treatment of several cancers, including metastatic colorectal cancer, non-squamous non-small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, and advanced cervical cancer. Amgen’s focus on developing biosimilars aims to provide more affordable and accessible treatment options for patients while maintaining high standards of quality and efficacy.
Merck
Company Profile
Merck, known as MSD outside the United States and Canada, is a global healthcare company with a rich history dating back to 1891. Headquartered in Rahway, New Jersey, Merck is dedicated to improving global health through innovative research and the development of high-quality medicines and vaccines. The company has a strong presence in pharmaceuticals, vaccines, and consumer care products, with operations spanning over 140 countries.
Business Overview
Merck’s business strategy emphasizes research and development, with a focus on addressing significant health challenges. The company operates in multiple therapeutic areas, including oncology, infectious diseases, and cardiovascular health. Merck’s commitment to innovation and collaboration has led to the development of numerous groundbreaking therapies and vaccines. The company’s business model includes strategic partnerships and acquisitions aimed at expanding its portfolio and enhancing its research capabilities.
Product Offered
Merck’s key product in the glioblastoma multiforme treatment market is TEMODAR® (Temozolomide). TEMODAR® is an oral alkylating agent used for the treatment of newly diagnosed glioblastoma multiforme and refractory anaplastic astrocytoma. It is administered in combination with radiation therapy and as maintenance therapy to extend patient survival. Merck’s ongoing research and development efforts aim to improve the efficacy and safety of TEMODAR® and explore new treatment options for brain tumors.

