1 Global GMP Plasmid DNA Manufacturing Market Size (Revenue) and CAGR (2024-2033)
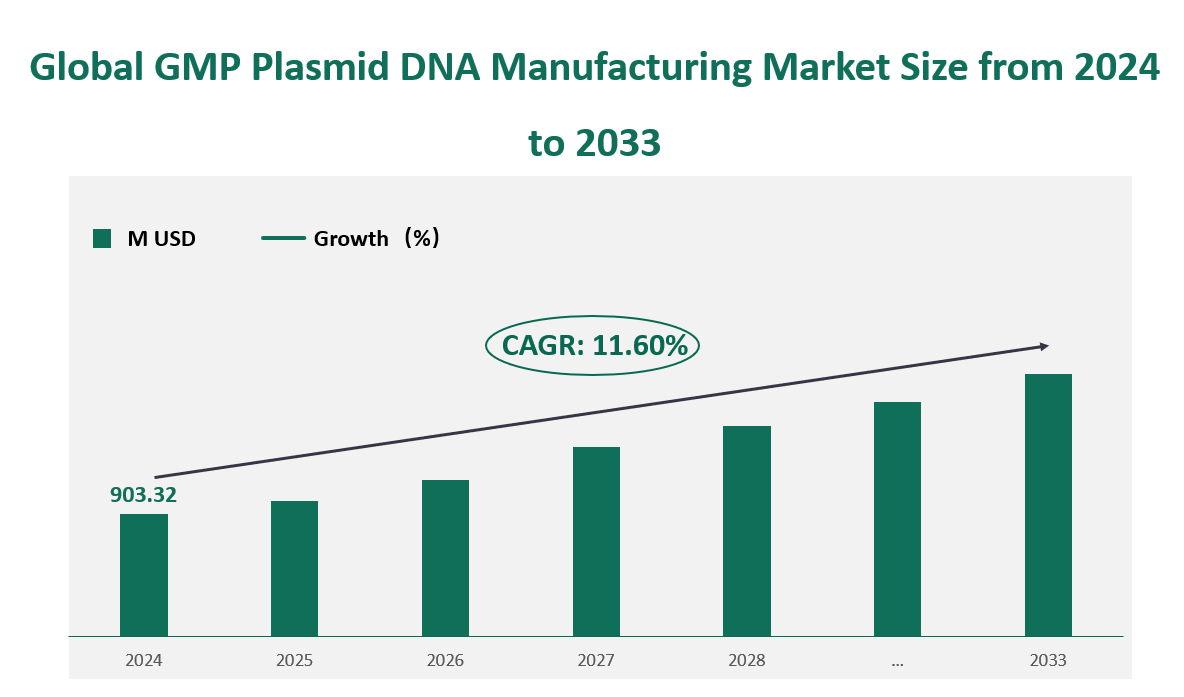
Global GMP Plasmid DNA Manufacturing market generated revenue of USD 903.32 Million in 2024 with a CAGR of 11.6% during 2024 to 2033.
The GMP Plasmid DNA Manufacturing market is currently experiencing significant growth, driven by several key factors. Firstly, the increasing prevalence of genetic disorders and the growing geriatric population have heightened the demand for advanced therapeutic solutions, including gene therapies and DNA vaccines. This has led to a surge in the need for high-quality GMP plasmid DNA as a critical component in these treatments.
Secondly, technological advancements in biopharmaceutical manufacturing have enabled more efficient and scalable production processes. Companies are investing heavily in optimizing their manufacturing capabilities to meet the rising demand while maintaining stringent quality standards. For instance, many manufacturers are adopting single-use technologies and automated systems to enhance efficiency and reduce contamination risks.
Figure Global GMP Plasmid DNA Manufacturing Market Size (M USD) Outlook (2024-2033)
2 GMP Plasmid DNA Manufacturing Drivers
Table Drivers
Item | Descriptions |
Rapid growth of the biopharmaceutical industry | In the past two years, the number of research new drugs has increased greatly, and many drugs have been authorized for commercial sale. Due to this growth, contract manufacturers providing GMP-grade plasmids are trying to keep up with the increasing demand. The recent epidemic situation has aggravated this problem, because many candidate vaccines are being developed, including DNA-based vaccines and mRNA-based vaccines, in which plasmid DNA is needed as the starting material of mRNA transcription in vitro. The continuous growth of downstream demand will promote the development of GMP Plasmid DNA Manufacturing industry. |
The increasing demand of aging population | Almost every country in the world is experiencing an increase in the number and proportion of the elderly in its population. According to the data of world population prospects: 2019 revision, by 2050, one sixth of the people in the world will be over 65 years old (16%), which is higher than one tenth (9%) in 2019. By 2050, a quarter of the population living in Europe and North America will be aged 65 or above. In 2018, the number of people aged 65 and over in the world exceeded that of children under 5 for the first time. The number of people aged 80 or above is expected to triple, from 143 million in 2019 to 426 million in 2050. The aging of the population will become an important driving force to promote the growth of the demand in the medical market, and people’s demand for vaccines, treatment is constantly increasing. In the past few years, gene and cell therapy has become an attractive treatment option for various diseases. In addition to common viral vectors, non-viral gene delivery methods using plasmid DNA are also very attractive. Gene immunization with plasmid DNA mimics the gene expression pathway of pathogens in cells and has been successfully applied to treat lower limb ischemia and cardiovascular regeneration, promising for the prevention of intractable diseases in modern medicine, including malaria, AIDS, hepatitis B and tuberculosis, through nucleic acid vaccines. Therefore, the increasing demand of aging population will promote the development of GMP Plasmid DNA Manufacturing industry. |
Growing popularity of gene therapy amongst the consumer/patients | More and more patients choose gene therapy, which is the main factor to promote the growth of GMP Plasmid DNA Manufacturing market. Gene therapy is a leading field of medical science, and it is expected to develop new treatment methods for patients with various diseases. Transgenic therapy has become a promising treatment for various diseases (mainly incurable diseases at present), including hereditary diseases and some viral infections. With the vigorous development of gene therapy, the demand for plasmid DNA is rapidly increasing. |
3 Global GMP Plasmid DNA Manufacturing Market by Type in 2024
The GMP Plasmid DNA Manufacturing market is segmented into three primary product types: Pre-Clinical Therapeutics, Clinical Therapeutics, and Marketed Therapeutics.
Pre-Clinical Therapeutics refers to the production of plasmid DNA used in the initial stages of drug development, including laboratory research and animal testing. In 2024, this segment had a market value of $65.06 million USD, accounting for approximately 7.2% of the total market share. While this segment is crucial for early-stage research, its market size is relatively smaller compared to other types due to the limited scope of pre-clinical studies.
Clinical Therapeutics involves the production of plasmid DNA for use in human clinical trials. This segment had the largest market share in 2024, with a value of $720.77 million USD, representing 79.8% of the total market. The significant market share of Clinical Therapeutics is attributed to the increasing number of clinical trials worldwide, driven by advancements in gene therapy and the growing demand for personalized medicine. The need for high-quality, GMP-compliant plasmid DNA in clinical trials ensures that this segment remains a dominant force in the market.
Marketed Therapeutics includes plasmid DNA used in commercially available treatments. In 2024, this segment had a market value of $117.49 million USD, accounting for 13.0% of the total market share. The growth of this segment is driven by the increasing number of approved gene therapies and DNA-based vaccines, which require consistent and large-scale production of plasmid DNA.
Table Global GMP Plasmid DNA Manufacturing Market Size and Share by Type in 2024
Type | Market Size (M USD) | Market Share (%) |
|---|---|---|
Pre-Clinical Therapeutics | 65.06 | 7.20 |
Clinical Therapeutics | 720.77 | 79.80 |
Marketed Therapeutics | 117.49 | 13.00 |
Total | 903.32 | 100.00 |
4 Global GMP Plasmid DNA Manufacturing Market by Application in 2024
The applications of GMP Plasmid DNA Manufacturing are diverse, with each application serving a unique purpose in the biopharmaceutical industry. The primary applications include Gene Therapy, DNA Vaccines, Immunotherapy, and Others.
Gene Therapy involves the use of plasmid DNA to introduce genetic material into cells to treat or prevent diseases. In 2024, the Gene Therapy segment had a market value of $319.13 million USD, accounting for 35.3% of the total market share. This segment is driven by the increasing prevalence of genetic disorders and the growing interest in gene-editing technologies such as CRISPR. The potential for gene therapy to provide long-term solutions for various diseases makes it a significant area of focus in the biopharmaceutical industry.
DNA Vaccines utilize plasmid DNA to produce antigens that stimulate an immune response. In 2024, this segment had a market value of $199.67 million USD, representing 22.1% of the total market share. The growth of this segment is fueled by the ongoing COVID-19 pandemic, which has accelerated the development and adoption of DNA-based vaccines. The advantages of DNA vaccines, such as their stability and ease of production, make them an attractive option for future vaccine development.
Immunotherapy involves the use of plasmid DNA to enhance the body’s immune response against diseases, particularly cancer. In 2024, the Immunotherapy segment had a market value of $85.98 million USD, accounting for 9.5% of the total market share. The growth of this segment is driven by the increasing incidence of cancer and the need for more effective treatments. Immunotherapy has shown promising results in clinical trials, leading to increased investment in this area.
Table Global GMP Plasmid DNA Manufacturing Market Size and Share by Application in 2024
Application | Market Size (M USD) | Market Share (%) |
|---|---|---|
Gene Therapy | 319.13 | 35.30 |
DNA Vaccines | 199.67 | 22.10 |
Immunotherapy | 85.98 | 9.50 |
Others | 298.54 | 33.10 |
Total | 903.32 | 100.00 |
5 Global GMP Plasmid DNA Manufacturing Market by Region in 2024
In 2024, the global GMP Plasmid DNA Manufacturing market exhibited significant regional disparities in terms of market size and growth rates. The market was dominated by North America, which accounted for the largest share of global revenue. This region’s prominence can be attributed to its robust biopharmaceutical industry, advanced healthcare infrastructure, and high demand for cutting-edge therapeutic solutions. In 2024, North America’s GMP Plasmid DNA Manufacturing market was valued at $417.04 million USD.
Europe followed closely behind North America, with a market value of $349.88 million USD in 2024. The European market’s strength is driven by its well-established pharmaceutical industry, stringent regulatory standards, and significant investments in research and development. The region’s focus on innovation and quality has positioned it as a key player in the global GMP Plasmid DNA Manufacturing market.
In contrast, emerging markets such as China and India showed remarkable growth potential despite their relatively smaller market sizes. China’s GMP Plasmid DNA Manufacturing market was valued at $57.06 million USD in 2024. The country’s rapid economic growth, increasing R&D investments, and expanding biopharmaceutical sector have fueled its market expansion. Similarly, India’s market was valued at $8.10 million USD in 2024. India’s market growth is driven by its large population, growing middle class, and increasing demand for affordable healthcare solutions.
Table Global GMP Plasmid DNA Manufacturing Market Size, Region Wise in 2024
Region | Market Size (M USD) |
|---|---|
North America | 417.04 |
Europe | 349.88 |
China | 57.06 |
Japan | 33.48 |
Middle East and Africa | 6.46 |
India | 8.10 |
South America | 6.69 |
South Korea | 10.13 |
Southeast Asia | 4.82 |
6 Global GMP Plasmid DNA Manufacturing Market Top 3 Players
Company Introduction and Business Overview: Aldevron is a leading provider of nucleic acid and protein production services, specializing in the manufacturing of GMP-grade plasmid DNA for research, preclinical, clinical, and commercial applications. Established in 1998, the company operates primarily in the United States and Europe. Aldevron is renowned for its high-quality products and stringent adherence to GMP standards, ensuring the highest quality and safety for its clients’ projects.
Products Offered: Aldevron offers a comprehensive range of GMP Plasmid DNA manufacturing services, including plasmid production for gene therapy, DNA vaccines, and other therapeutic applications. The company provides customized solutions tailored to meet the specific needs of its clients, from early research to commercial supply. Aldevron’s services include E. coli master and working cell bank generation, high-density fermentation, alkaline lysis, chromatographic purification, and quality control testing.
Revenue in 2021: In 2021, Aldevron reported a revenue of $229.01 million USD, making it the largest player in the GMP Plasmid DNA Manufacturing market. The company’s robust growth is driven by its extensive experience, advanced manufacturing facilities, and strong client relationships.
Company Introduction and Business Overview: Kaneka Eurogentec S.A., a subsidiary of Kaneka Corporation, is a global leader in the production of GMP-grade plasmid DNA. Established in 1985 and headquartered in Belgium, the company specializes in providing high-quality plasmid DNA for clinical trials and commercial applications. Eurogentec is certified by the US FDA and the European Medicines Agency (EMA), ensuring compliance with the highest regulatory standards.
Products Offered: Kaneka Eurogentec S.A. offers a wide range of GMP Plasmid DNA products, including plasmids for gene therapy, DNA vaccines, and other therapeutic applications. The company’s proprietary one-chromatography-step purification technology allows for high-yielding and high-purity plasmid DNA production. Eurogentec also provides custom plasmid DNA services, catering to the specific needs of its clients.
Revenue in 2021: In 2021, Kaneka Eurogentec S.A. reported a revenue of $93.37 million USD. The company’s growth is driven by its innovative technologies, extensive experience in GMP manufacturing, and strong market presence.
Company Introduction and Business Overview: Charles River Laboratories International, Inc. is a global provider of preclinical and clinical laboratory services, specializing in gene therapy and cell therapy solutions. Established in 1947, the company operates worldwide and offers a comprehensive range of services for the pharmaceutical, medical device, and biotechnology industries. Charles River’s acquisition of Cobra Biologics in 2021 further strengthened its position in the GMP Plasmid DNA Manufacturing market.
Products Offered: Charles River provides GMP Plasmid DNA manufacturing services, including plasmid production for clinical trials and commercial supply. The company’s services cover the entire spectrum of plasmid DNA production, from cell banking to final formulation and fill/finish. Charles River’s state-of-the-art facilities and stringent quality control measures ensure the highest standards of product quality and safety.
Revenue in 2021: In 2021, Charles River reported a revenue of $76.76 million USD from its GMP Plasmid DNA Manufacturing operations. The company’s growth is driven by its extensive service offerings, strong client relationships, and continuous investment in R&D and manufacturing capabilities.
Table Global GMP Plasmid DNA Manufacturing Revenue of Top3 Players in 2021
Company | 2021 |
Aldevron | 34.65% |
Kaneka Eurogentec S.A. | 14.13% |
Charles River | 11.61% |

