1 Global Sterilization Validation Service Market Outlook
The global Sterilization Validation Service market is projected to exhibit substantial growth in the coming years, with a CAGR of 13.26% from 2024 to 2033, reaching a total market size of $908.71 million USD in 2024. Sterilization validation services are crucial for ensuring the safety and efficacy of medical devices and pharmaceutical products. These services involve the selection and evaluation of the most suitable sterilization methods for products, such as steam sterilization, ethylene oxide (EtO) sterilization, electron beam radiation sterilization, and gamma sterilization. The process includes the development of a validation plan, execution of the plan, and submission of original data and final reports. This ensures that products meet the required sterility assurance levels (SAL) and are safe for use in healthcare settings.
Figure Global Sterilization Validation Service Market Size and Growth Rate (2024-2033)

2 Sterilization Validation Service Market Growth Drivers and Constraints
The growth of the sterilization validation service market is driven by several key factors. Firstly, the increasing incidence of hospital-acquired infections (HAIs) has heightened the awareness of the importance of sterilization. Inadequate sanitary conditions and poor infection control measures have led to a rise in HAIs, prompting healthcare providers to invest more in sterilization validation services. Secondly, the growing number of surgeries requiring high-intensity infection prevention and control is another significant driver. The aging population and the rise in chronic diseases have increased the demand for surgical procedures, thereby increasing the need for effective sterilization services.
However, the market also faces challenges. The use of ethylene oxide (EtO) in sterilization, for instance, has come under scrutiny due to its potential health risks and environmental impact. This has led to regulatory pressures and a shift towards alternative sterilization methods. Additionally, end-users not strictly adhering to sterilization standards can lead to insufficient sterilization, posing a risk of pathogen spread and hindering the market’s growth.
In conclusion, the global sterilization validation service market is poised for robust growth, driven by increasing healthcare demands and technological advancements. However, it must navigate challenges such as regulatory pressures and end-user compliance. Companies that invest in R&D, innovation, and strategic partnerships are likely to gain a competitive edge in this evolving market
3 Sterilization Validation Service Market Innovations and M&A Activities
The sterilization validation service market is witnessing significant technological innovations. Advanced aseptic processing technologies, such as isolators, closed restricted access barrier systems (RABS), blow-fill-seal (BFS), and form-fill-seal (FFS) technologies, are reducing the risk of human contamination. Automation and environmental design are improving the efficiency and reliability of sterilization processes. Moreover, the need for improved environmental surface cleaning and sterilization in healthcare facilities has gained recognition, driving innovation in this area.
Corporate mergers and acquisitions are also shaping the market landscape. For example, SteriPack Group acquired HS Design, enhancing its capabilities in medical product development and contract manufacturing. STERIS launched the Spordex® Self-Contained Biological Indicator (SCBI) Ampoules, offering faster and more reliable results for steam sterilization validation. These strategic moves not only strengthen the companies’ market positions but also drive the industry towards greater efficiency and innovation.
4 Global Sterilization Validation Service Market Analysis by Type
In 2024, the global sterilization validation service market is forecasted to have a total revenue of 908.71 million USD. The revenue market share for steam sterilization is expected to be 11.46%, with a revenue of 104.14 million USD. Ethylene oxide (EtO) sterilization is projected to hold a market share of 41.93%, with a revenue of 381.04 million USD. Electron beam radiation sterilization is anticipated to have a market share of 6.91%, with a revenue of 62.70 million USD. Gamma sterilization is expected to account for 32.20% of the market share, with a revenue of 292.65 million USD. Other types of sterilization services are forecasted to have a market share of 7.50%, with a revenue of 68.18 million USD. These figures indicate a diverse market with a significant portion dedicated to EtO and gamma sterilization, reflecting their widespread use and importance in the industry. The growth rates for these types of sterilization services suggest a dynamic market with varying levels of expansion, influenced by factors such as technological advancements, regulatory requirements, and the evolving needs of healthcare providers.
Table Global Sterilization Validation Service Market Size and Share by Type in 2024
Type | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Steam Sterilization | 104.14 | 11.46% |
Ethylene Oxide (EtO) Sterilization | 381.04 | 41.93% |
Electron Beam Radiation Sterilization | 62.70 | 6.91% |
Gamma Sterilization | 292.65 | 32.20% |
Others | 68.18 | 7.50% |
5 Global Sterilization Validation Service Market Analysis by Application
In 2024, the global sterilization validation service market is forecasted to have a total value of 908.71 million USD. The value market share for hospitals & clinics is expected to be 45.13%, with a value of 410.10 million USD. Medical device companies are projected to hold a market share of 29.97%, with a value of 273.43 million USD. Pharmaceuticals are anticipated to have a market share of 15.79%, with a value of 143.49 million USD. Other applications are forecasted to account for 9.11% of the market share, with a value of 81.69 million USD. These figures highlight the significant role of hospitals & clinics in the market, followed by medical device companies and pharmaceuticals. The growth rates for these applications suggest a robust market with varying levels of expansion, driven by factors such as increasing healthcare demands, technological advancements in sterilization methods, and the growing importance of infection control in various healthcare settings.
Table Global Sterilization Validation Service Market Size and Share by Application in 2024
Application | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Hospitals & Clinics | 410.10 | 45.13% |
Medical Device Companies | 273.43 | 29.97% |
Pharmaceuticals | 143.49 | 15.79% |
Others | 81.69 | 9.11% |
6 Global Sterilization Validation Service Market Analysis by Region
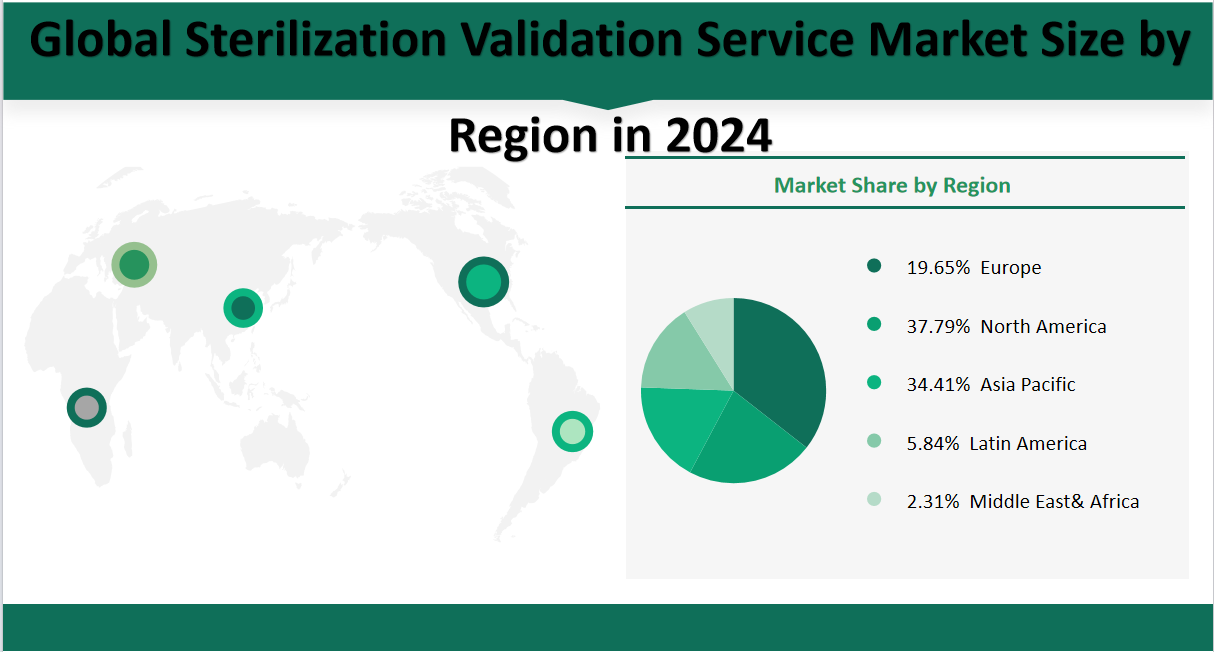
In 2024, the global sterilization validation service market is forecasted to reach a value of 908.71 million USD. The market share distribution by region is as follows: North America is expected to hold the largest share at 37.79%, valued at 343.41 million USD. Europe follows with a market share of 19.65%, valued at 178.52 million USD. The Asia-Pacific region is anticipated to have a significant share of 34.41%, valued at 312.73 million USD. Latin America is projected to account for 5.84% of the market, valued at 53.06 million USD. Lastly, the Middle East & Africa region is expected to have a market share of 2.31%, valued at 20.99 million USD. This distribution indicates a diverse market with North America and the Asia-Pacific region leading in terms of value, reflecting their strong healthcare infrastructure and growing demand for sterilization services..
Figure Global Sterilization Validation Service Market Share by Region in 2024
7 Top 3 Companies of Global Sterilization Validation Service Market
7.1 STERIS
Company Introduction and Business Overview:
STERIS is a leading global provider of infection prevention and contamination control products and services. With a unique combination of offerings, STERIS delivers customized solutions for a variety of environments, including operating rooms, sterile processing departments, GI/endoscopy suites, and ambulatory surgery centers. The company is committed to helping healthcare facilities gain productivity, lower costs, and protect staff and patients through its comprehensive range of products and services.
Products Offered:
STERIS offers a wide range of sterilization validation products, including:
Steam Sterilization: STERIS provides biological indicators, chemical indicators, and Bowie-Dick test kits designed to provide key information about the sterilization process.
Ethylene Oxide (EtO) Sterilization: STERIS offers a variety of EtO sterilization solutions, ensuring that medical devices and equipment are effectively sterilized.
Electron Beam Radiation Sterilization: STERIS utilizes advanced electron beam technology to sterilize products, offering a safe and efficient alternative to traditional methods.
Gamma Sterilization: STERIS provides gamma sterilization services, which are widely used for disposable medical devices and other products requiring high levels of sterility.
Sales Revenue in the Latest Year:
In the latest year, STERIS reported a revenue of 112.19 million USD from its sterilization validation service segment. This revenue reflects the company’s strong market position and its ability to deliver high-quality, technologically advanced solutions to its customers.
7.2 Cantel Medical
Company Introduction and Business Overview:
Cantel Medical is a global company dedicated to delivering innovative infection prevention products and services for patients, caregivers, and other healthcare providers. The company’s products and solutions are designed to improve outcomes and help save lives. Cantel Medical offers an expansive portfolio of endoscopy, water purification and filtration, and healthcare disposables, providing high-quality infection prevention solutions and unparalleled service.
Products Offered:
Cantel Medical’s product offerings include:
CANEXIS™ Sterilization Solution: This comprehensive solution provides a modular suite of applications to support proactive management and planning for central sterile services departments in hospitals.
Endoscopy Products: Cantel Medical offers a range of endoscopy products, including scopes, accessories, and reprocessing equipment, designed to ensure the highest standards of infection control.
Water Purification and Filtration: The company provides advanced water purification and filtration systems, essential for maintaining the quality of water used in healthcare settings.
Healthcare Disposables: Cantel Medical offers a variety of disposable products, such as gowns, gloves, and masks, which are crucial for infection prevention.
Sales Revenue in the Latest Year:
In the latest year, Cantel Medical reported a revenue of 79.17 million USD from its sterilization validation service segment. This revenue highlights the company’s growth and its commitment to providing innovative and effective infection prevention solutions.
7.3 Cretex Companies
Company Introduction and Business Overview:
Cretex Companies is a family of companies that provides manufacturing and engineering services to medical device original equipment manufacturers (OEMs). The company offers a complete range of end-to-end manufacturing capabilities, leveraging the latest technologies and talented professionals to meet any manufacturing or engineering challenge. Cretex Companies is known for its commitment to quality and innovation, ensuring that its products and services meet the highest standards.
Products Offered:
Cretex Companies’ product offerings include:
Pre-Validated EO Sterilization: Cretex Companies provides a pre-verified EO cycle through its QSTERILE™ program, which eliminates the need for customers to perform their own complete EO sterilization validation, saving time and money.
Custom Manufacturing Solutions: The company offers custom manufacturing services, including injection molding, assembly, and packaging, tailored to meet the specific needs of medical device OEMs.
Engineering Services: Cretex Companies provides engineering services to support the design and development of new products, ensuring that they meet regulatory requirements and customer specifications.
Quality Assurance: The company emphasizes quality assurance, providing comprehensive testing and validation services to ensure that products are safe and effective for use.
Sales Revenue in the Latest Year:
In the latest year, Cretex Companies reported a revenue of 54.46 million USD from its sterilization validation service segment. This revenue demonstrates the company’s strong performance in the market and its ability to provide high-quality, customized solutions to its customers.

