1 Global HPV Testing Kits Market Insight Analysis
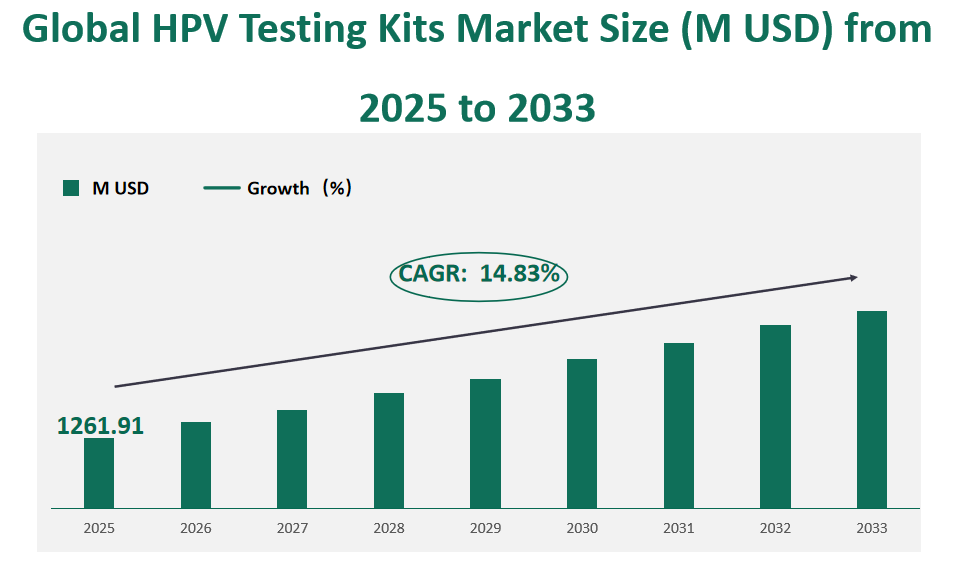
The global HPV testing kits market size is expected to reach 1261.91 million in 2025, with a CAGR of 14.83% from 2025 to 2033.
HPV test kits are products used to detect the presence of the human papillomavirus (HPV) in the body. HPV is a common sexually transmitted infection that can cause cervical and other types of cancer, as well as genital warts. These kits typically use samples of cells or fluid from the cervix, vagina, or anus, and then test those samples for the presence of certain types of HPV. HPV test kits can be used to diagnose HPV infection, monitor disease progression, or evaluate treatment response. These tests can be done at home or in a healthcare facility and can help identify individuals who may be at risk for HPV-related health problems.
Figure Global HPV Testing Kits Market Size (M USD) and CAGR (2025-2033)
2 HPV Testing Kits Market Growth Drivers and Restraints
Disease prevalence drive: Cervical cancer is one of the most common cancers among women worldwide. According to the World Health Organization, there were approximately 570,000 newly diagnosed cases of cervical cancer worldwide in 2018, more than 500,000 women were infected with cervical cancer in 2020, and approximately 342,000 women died from it. The American Cancer Society estimated in 2022 that there will be nearly 14,100 new cases of invasive cervical cancer in the United States. The large number of cervical cancer cases has led to an increasing demand for HPV testing and PAP testing, which has strongly promoted the growth of the HPV testing kit market.
Increased awareness of testing: HPV, as a common sexually transmitted infection virus, can cause a variety of cancers, and the incidence of related cancers has been on the rise in recent years. Women and medical professionals are increasingly aware of the importance of regular HPV screening to prevent cervical cancer. At the same time, governments and non-profit organizations are actively carrying out publicity activities and expanding screening programs, such as the US Affordable Care Act, which requires coverage of HPV testing and Pap smear testing, which have led to continued growth in market demand for HPV testing kits.
Strict regulations and policies: Medical products are strictly regulated, and HPV test kits must meet different regulatory requirements in different countries, such as the supervision of the US FDA, the European EMA, and the Chinese NMPA. Companies need to invest a lot of resources to ensure product compliance, which increases the difficulty and cost of product development and listing. If they fail to adapt to regulatory changes in a timely manner, products may not be able to enter the market or face recall risks, limiting the speed of market expansion.
Intensified market competition: The HPV test kit industry is developing rapidly, and the broad market prospects have attracted a large amount of capital influx, industry integration has accelerated, and market competition has become increasingly fierce. Many manufacturers and suppliers are participating in the competition, and product homogeneity is gradually emerging.
At the same time, consumers have higher and higher requirements for products in terms of price, quality, accuracy, ease of use, and privacy protection. Companies need to continuously improve the quality of products and services to meet demand, which places higher demands on the company’s R&D capabilities, production efficiency, and marketing capabilities. Some companies with weaker competitiveness may be eliminated in the competition, affecting the overall development of the market.
3 Technological Innovations in the HPV Testing Kits Market
Diverse development of detection technology: Currently, HPV detection technologies are rich and diverse, and commonly used ones include the second-generation hybrid capture method, fluorescent PCR method, enzyme switching signal amplification method and mRNA detection method. The second-generation hybrid capture method is based on liquid-phase HPV DNA-RNA probe hybridization and isotope signal amplification system, which can detect multiple HPV genotypes with high sensitivity and specificity;
the fluorescent PCR method is easy to operate and the results are visualized; the enzyme switching signal amplification method can identify target DNA and generate fluorescent signals based on specific principles. With the development of science and technology, these technologies are constantly improved and perfected, providing more accurate, fast and convenient detection methods for clinicians, which helps to diagnose and monitor cervical cancer earlier.
New detection forms emerge: Home HPV testing has become a new trend. It has the advantages of convenience, privacy protection, and increased screening rates. It meets the needs of some people who cannot go to the hospital for screening due to time or other factors, making the screening and management of HPV infection more popular and efficient. In addition, new forms such as urine testing are also developing, which have the characteristics of high compliance and convenient sample collection, providing more options for cervical cancer prevention and control.
Adjustment of corporate strategic layout: Enterprises optimize resource allocation, expand business areas and enhance market competitiveness through mergers and acquisitions. For example, Roche’s acquisition of Stratos Genomics aims to further develop DNA-based sequencing technology for diagnosis. The new technology acquired is expected to bring faster, more flexible and cost-effective solutions to clinical diagnosis;
BD’s acquisition of Parata Systems has advanced its layout in the field of pharmacy automation solutions and enhanced its competitiveness in the medical technology market; Hologic’s acquisition of Bolder Surgical has broadened its surgical product portfolio, incorporated advanced energy vascular sealing surgical equipment into its business scope, and enhanced its comprehensive strength in the field of women’s health.
4 Global HPV Testing Kits Market Size by Type
At-home HPV testing kits are designed for self-collection and use, allowing individuals to perform tests in the privacy and convenience of their own homes. This type of kit is particularly appealing due to its accessibility and privacy, making it an attractive option for those who prefer not to visit healthcare facilities. According to the forecast, the At-home segment is projected to generate a revenue of $181.51 million USD in 2025, capturing a market share of 14.38% of the total HPV Testing Kits market.
The growth in the At-home segment is driven by several factors, including increasing consumer awareness of HPV and its associated health risks, as well as the demand for convenient and private testing options. Additionally, advancements in self-sampling technologies have made these kits more reliable and user-friendly, further boosting their adoption rates.
Hospital-use HPV testing kits are designed for professional use in clinical settings. These kits are typically more comprehensive and are used for routine screenings, diagnostics, and monitoring treatment efficacy. In 2025, the Hospital Use segment is expected to generate a revenue of $1,080.40 million USD, accounting for a market share of 85.62% of the total market share.
Hospital-use kits are essential for healthcare providers to conduct accurate and reliable HPV screenings, particularly for high-risk populations. The high sensitivity and specificity of these kits make them ideal for detecting HPV infections early, allowing for timely interventions and improved patient outcomes. The significant market share of hospital-use kits reflects their critical role in healthcare systems worldwide.
Table Global HPV Testing Kits Market Size and Share by Type in 2025
Type | Market Size (M USD) 2025 | Market Share 2025 |
|---|---|---|
At-home | 181.51 | 14.38% |
Hospital Use | 1080.40 | 85.62% |
5 Global HPV Testing Kits Market Size by Application
The two main applications in the HPV testing kits market are Cervical Cancer Screening and Vaginal Cancer Screening & Others. Cervical Cancer Screening is the dominant application. In 2025, its market revenue is forecasted to be 1130.28 million USD. This accounts for a substantial market share of 89.57%. The high revenue and market share for Cervical Cancer Screening can be attributed to several factors.
Cervical cancer is a major health concern among women globally. It is one of the most common cancers in women, and HPV is a well – known risk factor for this disease. As a result, there is a high demand for screening tests to detect HPV in the context of cervical cancer prevention. Regular screening programs, awareness campaigns, and medical guidelines that recommend HPV testing for cervical cancer prevention have all contributed to this significant market presence.
On the other hand, the application of Vaginal Cancer Screening & Others has a projected market revenue of 131.64 million USD in 2025. This application holds a market share of 10.43%. Although it has a relatively smaller share compared to Cervical Cancer Screening, it still plays an important role in the overall HPV testing kits market. Vaginal cancer, while less common than cervical cancer, still poses a threat to women’s health. Additionally, the “Others” category may include screening for other HPV – related conditions or applications in research and monitoring, which also contribute to the market revenue.
Table Global HPV Testing Kits Market Size and Share by Application in 2025
Application | Market Size (M USD) 2025 | Market Share 2025 |
|---|---|---|
Cervical Cancer Screening | 1130.28 | 89.57% |
Vaginal Cancer Screening & Others | 131.64 | 10.43% |
6 Global HPV Testing Kits Market Size by Region
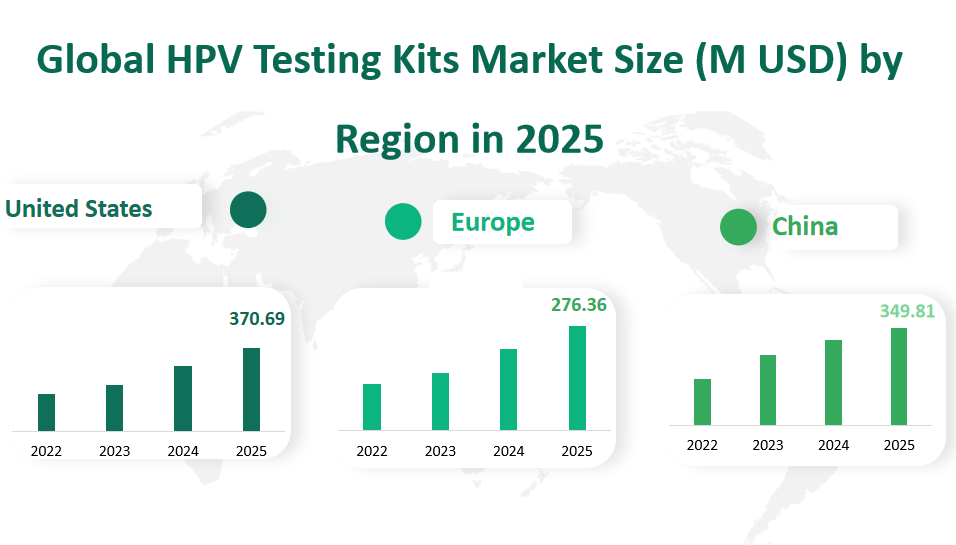
In the United States, the HPV Testing Kits market is projected to reach a revenue of $370.69 million USD by 2025. The market has been growing steadily, reflecting the country’s advanced healthcare infrastructure and the high awareness among consumers about the importance of regular health screenings. The United States has been at the forefront of implementing public health policies that promote HPV vaccinations and screenings, which has significantly contributed to the market’s expansion.
The growth in the U.S. market can also be attributed to the presence of leading healthcare companies that manufacture and distribute HPV testing kits. Additionally, the country’s significant investment in medical research and development ensures that innovative and accurate testing methods are continuously introduced to the market.
Europe is expected to see a market revenue of $276.36 million USD for HPV Testing Kits in 2025. The European market is characterized by a strong emphasis on preventive healthcare measures, with many countries offering HPV vaccinations as part of their national health programs. This has led to a robust demand for HPV testing kits, as both vaccinated and unvaccinated individuals seek regular screenings.
The European market also benefits from the presence of several key players in the HPV Testing Kits industry, contributing to the region’s market growth through their innovative products and extensive distribution networks. The European Union’s efforts to harmonize health policies across member states further support the market’s expansion by ensuring a consistent approach to HPV screening and vaccination.
China is projected to achieve a market revenue of $349.81 million USD for HPV Testing Kits in 2025. The market in China has been rapidly growing due to the country’s large population base and the increasing focus on improving public health standards. The Chinese government’s initiatives to enhance healthcare access and quality have played a crucial role in boosting the demand for HPV testing kits.
Moreover, China’s ongoing efforts to raise awareness about HPV and its associated risks, coupled with the expansion of its healthcare sector, are driving the market’s growth. The country’s strategic investments in healthcare infrastructure and its commitment to public health initiatives are expected to further propel the market’s expansion in the coming years.
Figure Global HPV Testing Kits Market Size (M USD) by Region in 2025
7 Global HPV Testing Kits Market Analysis by Major Players
Hoffmann-La Roche Ltd
Company Profile: Hoffmann-La Roche Ltd, established in 1896, is headquartered in Switzerland. It operates globally, offering a wide range of diagnostic solutions.
Business Overview: Roche Diagnostics, a division of Hoffmann-La Roche, develops and integrates diagnostic solutions that address current challenges and anticipate future needs. They offer comprehensive in vitro diagnostics solutions across molecular diagnostics, clinical chemistry, immunoassays, tissue diagnostics, point of care testing, patient self-testing, next-generation sequencing, and laboratory automation and IT solutions.
Product Offered: Their HPV Testing Kits include the cobas® HPV Test, which provides 3-in-1 HPV test results. This test is known for its high sensitivity and specificity, validated for detection of >CIN2 lesions, and aligns with international guidelines for HPV testing in cervical screening purposes.
2023 Revenue Summary: Hoffmann-La Roche Ltd led the market in 2023 with a revenue of $191.99 million USD, showcasing its strong position in the HPV Testing Kits industry.
Guangdong Hybribio
Company Profile: Guangdong Hybribio, founded in 2003, is based in China and sells its products worldwide.
Business Overview: Guangdong Hybribio Biotech Co. Ltd. specializes in the manufacture and distribution of biotechnology products, including molecular diagnostic reagents, formaldehyde assay kits, parting detection kits, gene detection kits, stem cell identification agents, and more.
Product Offered: They offer a Human papillomavirus (23 types) nucleic acid typing detection kit that uses fluorescent PCR method. This kit is known for accurate typing and hierarchical management, high sensitivity and specificity, large throughput, various types of detection, quick and easy operation, and high automation.
2023 Revenue Summary: Guangdong Hybribio achieved a revenue of $191.02 million USD in 2023, marking it as a significant player in the global HPV Testing Kits market.
Hologic, Inc.
Company Profile: Hologic, Inc., established in 1985, is headquartered in the USA and operates worldwide.
Business Overview: Hologic, Inc. is primarily focused on women’s health, selling medical devices for diagnostics, surgery, and medical imaging.
Product Offered: Their HPV Testing Kits include the ThinPrep Pap test and the Aptima HPV assays. These tests help detect the presence of abnormal cervical cells and identify high-risk HPV mRNA indicative of infections likely to lead to cervical disease.
2023 Revenue Summary: Hologic, Inc. generated a revenue of $103.28 million USD in 2023, solidifying its position among the top HPV Testing Kits providers globally.

