1. Global Mono Hepatitis A Vaccine Market Insight Analysis
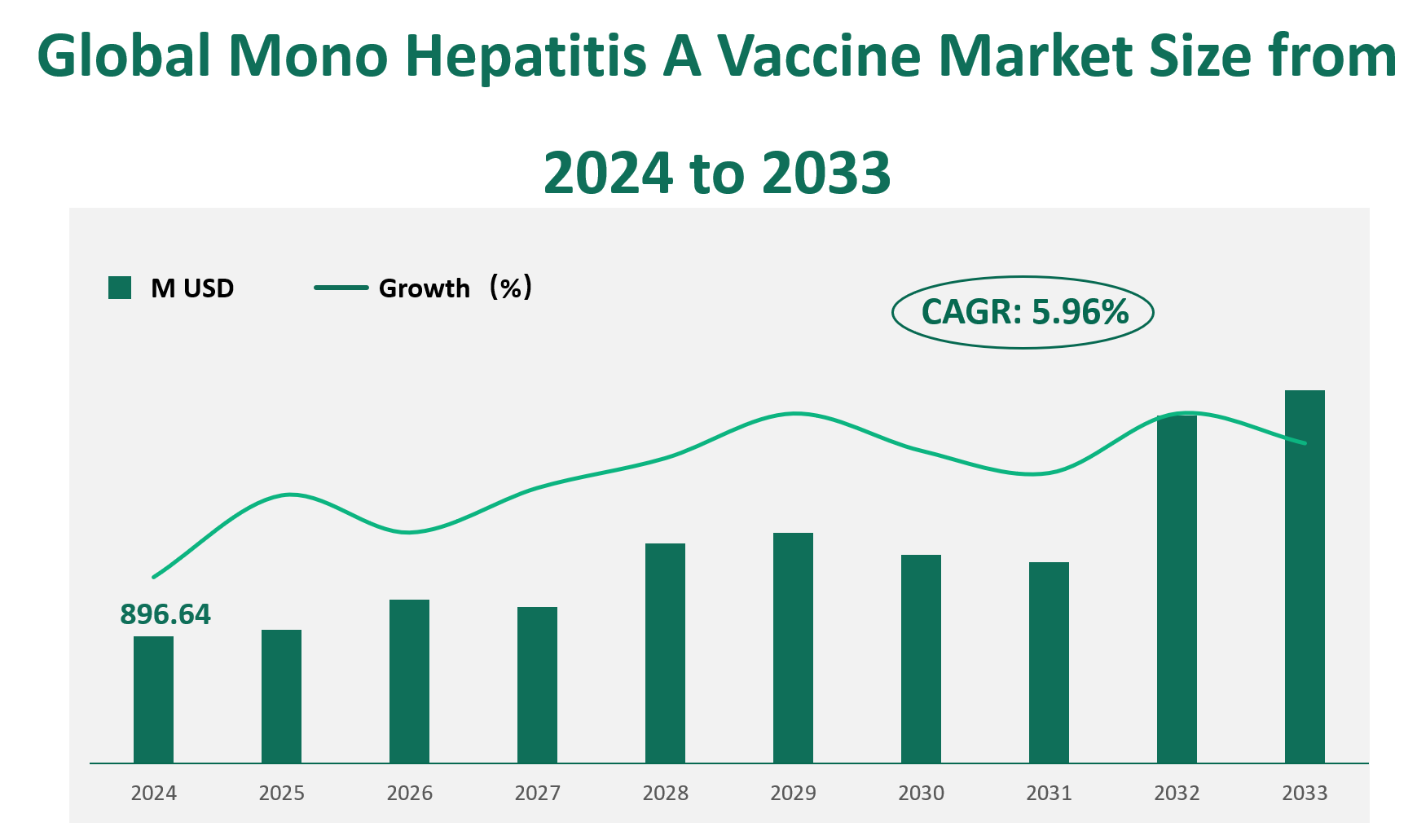
The global Mono Hepatitis A Vaccine market is projected to reach a revenue of 896.64 million USD in 2024, with a compound annual growth rate (CAGR) of 5.96% from 2024 to 2033.
The Mono Hepatitis A Vaccine is a critical tool in preventing Hepatitis A, a viral liver infection caused by the Hepatitis A virus (HAV). This vaccine is essential for individuals in regions with high prevalence of the disease, travelers to endemic areas, and those at high risk of exposure. The vaccine is available in two primary forms: inactivated and live attenuated. Inactivated vaccines are composed of virus particles that have been rendered non-infectious, while live attenuated vaccines contain weakened forms of the virus that can still replicate but do not cause disease.
Figure Global Mono Hepatitis A Vaccine Market Size (M USD) and CAGR (2024-2033)
2. Driving and Limiting Factors of Mono Hepatitis A Vaccine Market Growth
The growth of the Mono Hepatitis A Vaccine market is propelled by several key drivers. Firstly, the increasing prevalence of Hepatitis A in developing regions, coupled with high birth rates, creates a significant demand for vaccines. The World Health Organization (WHO) estimates that approximately 1.5 million people are infected with Hepatitis A annually, highlighting the need for widespread vaccination. Additionally, improved healthcare infrastructure and increased government support for immunization programs are driving market growth. For instance, 37 countries worldwide have included the Hepatitis A vaccine in their national immunization lists, further boosting demand.
However, the market also faces several limiting factors. One of the primary challenges is the strict regulatory environment surrounding vaccine production and distribution. Vaccines are complex biological products, and their production, circulation, and use are subject to stringent regulations to ensure safety and efficacy. This complexity creates high barriers to entry for new market participants.
Another significant challenge is the decline in global birth rates, particularly in developed countries. As birth rates stabilize or decline, the market for Hepatitis A vaccines may become saturated, reducing growth opportunities. Furthermore, the high costs associated with vaccine research, development, and distribution can limit market expansion.
3. Technology Innovation and Corporate Mergers and Acquisitions in Mono Hepatitis A Vaccine Market
Technological innovation plays a crucial role in the Mono Hepatitis A Vaccine market. Advances in vaccine development have led to the creation of more effective and safer vaccines. For example, the development of inactivated vaccines has provided a safer alternative to live attenuated vaccines, especially for immunocompromised individuals. Additionally, improvements in vaccine delivery systems, such as needle-free injections and temperature-stable formulations, have enhanced the accessibility and usability of Hepatitis A vaccines in remote and underserved areas.
Corporate mergers and acquisitions (M&A) have also significantly impacted the market. These activities have led to the consolidation of market players, enhancing their research and development capabilities and expanding their market reach. For instance, MSD’s acquisition of Themis in 2020 demonstrates the strategic importance of M&A in strengthening a company’s position in the vaccine market. Similarly, Sanofi’s acquisition of Shantha Biotechnics in 2013 and GSK’s acquisition of Novartis’ vaccines business in 2015 highlight the trend of large pharmaceutical companies expanding their vaccine portfolios through strategic acquisitions.
4. Global Mono Hepatitis A Vaccine Market Size by Type
The Mono Hepatitis A Vaccine market is segmented into two primary product types: Inactivated Vaccine and Live Attenuated Vaccine.
Inactivated vaccines are composed of virus particles, bacteria, or other pathogens that have been grown in culture and then rendered non-infectious. These vaccines cannot cause the disease but can still stimulate an immune response. Inactivated vaccines are often used for diseases where live vaccines might pose a risk or where the pathogen is difficult to attenuate safely. The market revenue for Inactivated Hepatitis A Vaccine in 2024 is forecasted to be 748.97 M USD. This type of vaccine has been widely adopted due to its safety profile and efficacy, making it a preferred choice in many regions, especially where healthcare infrastructure is robust.
Live attenuated vaccines are prepared from live microorganisms that have been weakened under laboratory conditions. These vaccines replicate and produce an immune response in the vaccinated individual, usually without causing disease. Live attenuated vaccines can provide strong and long-lasting immunity but require careful handling and storage to maintain their viability. The market revenue for Live Attenuated Hepatitis A Vaccine in 2024 is forecasted to be 147.67 M USD.
Table Global Mono Hepatitis A Vaccine Market Size by Type in 2024
5. Global Mono Hepatitis A Vaccine Market Size by Application
The Mono Hepatitis A Vaccine market is also segmented by application, with the primary applications being Government Institutions, Private Sector, and Other applications.
Government Institutions include public health departments, hospitals, and clinics that provide vaccines as part of national immunization programs. These institutions play a crucial role in ensuring widespread vaccination coverage, especially in developing countries where hepatitis A is endemic. The market revenue for Mono Hepatitis A Vaccine used in Government Institutions in 2024 is forecasted to be 483.83 M USD. Government-led vaccination programs are essential in controlling the spread of hepatitis A and reducing its incidence in high-risk populations.
The Private Sector includes private hospitals, clinics, and healthcare providers that offer vaccines to patients. This sector often caters to individuals seeking additional healthcare services beyond what is provided by the government. The market revenue for Mono Hepatitis A Vaccine in the Private Sector in 2024 is forecasted to be 389.36 M USD.
Table Global Mono Hepatitis A Vaccine Market Size by Application in 2024
Application | Market Size (M USD) 2024 |
Government Institution | 483.83 |
Private Sector | 389.36 |
Other | 23.45 |
6. Global Mono Hepatitis A Vaccine Market by Top Regions
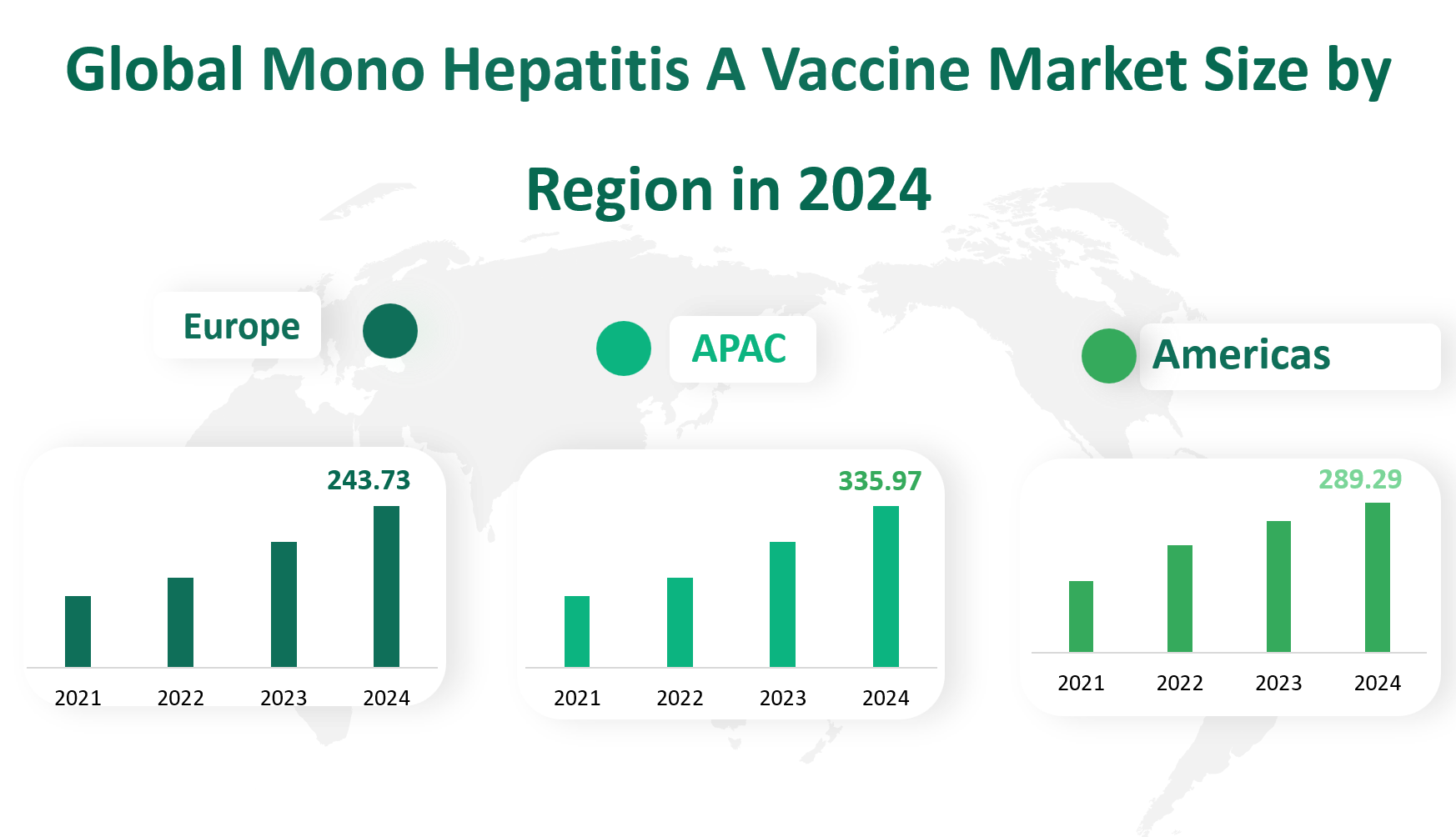
The market revenue for Mono Hepatitis A Vaccine in America is forecasted to be 289.29 M USD. America, comprising the United States, Canada, Mexico, and Brazil, has a robust healthcare infrastructure and high awareness levels regarding hepatitis A. The U.S. is the largest market within this region, driven by strong government support for vaccination programs and a high demand for safe and effective vaccines. Canada and Mexico also contribute significantly, with growing healthcare sectors and increasing focus on preventive healthcare.
The market revenue for Mono Hepatitis A Vaccine in the Asia-Pacific region is forecasted to be 335.97 M USD. The Asia-Pacific region is the largest market for Mono Hepatitis A Vaccine, driven by its large population base and growing healthcare sector. China and Japan are the key markets within this region, with significant demand for vaccines due to high hepatitis A incidence rates. South Korea, Australia, and India also contribute to the market growth, with increasing government initiatives to improve public health and vaccination coverage.
The market revenue for Mono Hepatitis A Vaccine in Europe is forecasted to be 243.73 M USD. Europe, comprising Germany, France, the UK, Italy, and Russia, has a well-developed healthcare system and high vaccination coverage rates. The market is driven by stringent regulatory standards and a focus on public health initiatives. Germany and the UK are the largest markets within this region, with significant contributions from France and Italy.
The market revenue for Mono Hepatitis A Vaccine in the Middle East & Africa is forecasted to be 27.64 M USD. The Middle East & Africa region includes Egypt, South Africa, Saudi Arabia, Israel, Turkey, and the GCC countries. This region is characterized by a growing healthcare sector and increasing awareness of hepatitis A. Egypt and South Africa are the key markets, with significant demand for vaccines due to high hepatitis A incidence rates.
Figure Global Mono Hepatitis A Vaccine Market Size by Region in 2024
7. Global Mono Hepatitis A Vaccine Market Analysis by Major Players
7.1 MSD
Company Introduction and Business Overview: MSD (Merck & Co., Inc.) is a research-based healthcare company with a focus on developing innovative therapeutic solutions. Established in 1963, MSD operates globally and is known for its extensive research and development in various medical areas, including infectious diseases.
Products Offered: MSD offers the VAQTA vaccine, which is indicated for the prevention of disease caused by hepatitis A virus (HAV) in individuals aged 12 months and older.
7.2 Sanofi
Company Introduction and Business Overview: Sanofi is a global healthcare company engaged in the research, development, manufacturing, and marketing of innovative therapeutic solutions. Established in 2004, Sanofi operates primarily in North America and Europe, with a focus on diabetes solutions, human vaccines, and innovative drugs.
Products Offered: Sanofi offers the AVAXIM vaccine, which is indicated for active immunization against infection caused by hepatitis A virus in susceptible adults and adolescents aged 16 years and above.
7.3 GSK
Company Introduction and Business Overview: GSK (GlaxoSmithKline) is a science-led global healthcare company with a broad portfolio of innovative drugs and mature drug products. Established in 1929, GSK operates primarily in Europe, the Americas, and Asia, with a focus on respiratory and HIV treatments.
Products Offered: GSK offers the HAVRIX vaccine, which is indicated for active immunization against disease caused by hepatitis A virus in individuals aged 12 months and older.

