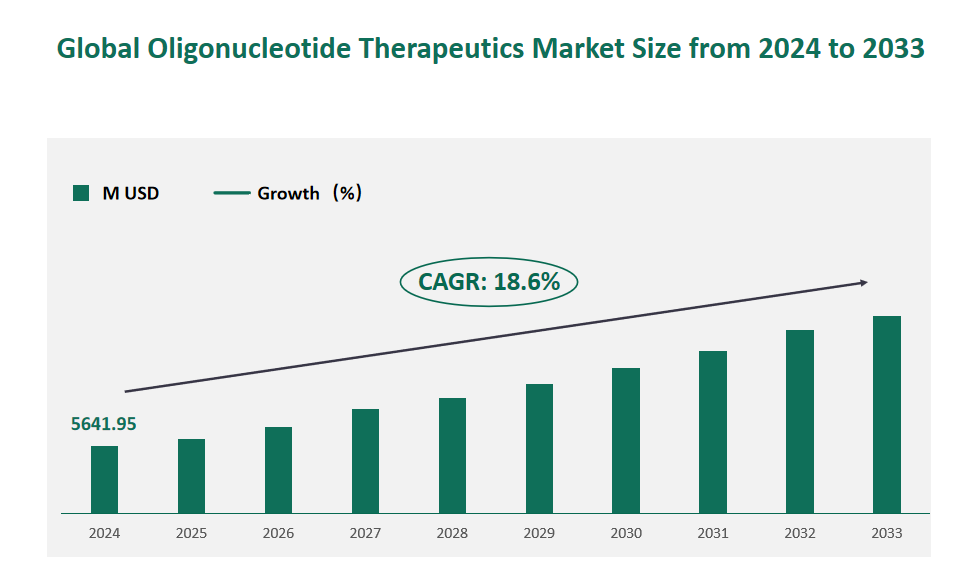
1 Global Oligonucleotide Therapeutics Market Size (Value) and CAGR (2024-2033)
In 2024, the global Oligonucleotide Therapeutics market was valued at USD 5641.95 million, with a CAGR of 18.6% from 2024 to 2033.
Oligonucleotide therapeutics are a class of advanced, molecular-targeted agents that utilize chemically synthesized oligonucleotides with a single-stranded deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) backbone. These agents can specifically bind to target genes or proteins, inhibiting gene expression or impeding protein function. This specificity allows them to target molecules that are not controllable by conventional drugs, such as mRNA or noncoding RNA. As a result, oligonucleotide therapeutics have emerged as a powerful tool in the development of innovative treatments for various cancers and genetic diseases.
Figure Global Oligonucleotide Therapeutics Market Size (M USD) and CAGR 2024-2033
2 Oligonucleotide Therapeutics Market Drivers
The Oligonucleotide Therapeutics market is experiencing significant growth, driven by several key factors that are reshaping the landscape of modern medicine. One of the primary drivers is the increasing prevalence of genetic disorders and complex diseases that require targeted treatments. Conditions such as spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS), and hereditary transthyretin-mediated amyloidosis (hATTR) are driving the demand for more effective therapies. Oligonucleotide therapeutics offer a unique advantage by targeting specific genetic sequences, enabling the development of treatments that conventional drugs cannot achieve.
Another significant driver is the continuous advancement in drug delivery systems. Researchers are developing innovative methods to deliver oligonucleotides directly to target tissues and cells, enhancing their efficacy and reducing side effects. For instance, liposomes and nanoparticles are being utilized to improve the pharmacokinetics and stability of these therapeutic agents. These advancements are crucial in overcoming the challenges associated with the delivery of large molecules, such as oligonucleotides, to specific sites within the body.
3 Oligonucleotide Therapeutics Market Restraints
Despite the promising growth prospects, the Oligonucleotide Therapeutics market faces several challenges and restraints that could hinder its expansion. One of the primary challenges is the complex regulatory environment. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) impose stringent guidelines for the development and approval of new therapies. These regulations ensure the safety and efficacy of treatments but also add to the time and cost required for bringing new products to market.
Another significant challenge is the high cost associated with the research and development of oligonucleotide therapeutics. Developing these advanced therapies requires substantial investment in R&D, clinical trials, and manufacturing processes. The high costs can be a barrier for smaller companies and startups, limiting the number of players in the market and potentially slowing down innovation.
4 Global Oligonucleotide Therapeutics Market Size by Type in 2024
Antisense Oligonucleotides (ASOs) are expected to hold the largest market share in 2024, with a projected value of $3470.41 million. ASOs are short, synthetic, single-stranded oligodeoxynucleotides that can modulate gene expression by binding to specific RNA sequences. This mechanism allows them to reduce, restore, or modify protein expression, making them highly effective in treating genetic disorders. ASOs have been successfully used in therapies for conditions such as spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD). The growth of this segment is driven by the increasing number of approvals for ASO-based therapies and the ongoing development of new indications.
Small Interfering RNA (siRNA) is another significant segment, projected to reach a market value of $1869.88 million in 2024. siRNA therapies rely on sequence complementarity between RNAs and target mRNAs to inhibit their activity, effectively silencing specific genes. This technology has shown great potential in treating a wide range of diseases, from cancer to genetic disorders. The development of siRNA therapies has been accelerated by advancements in delivery systems, such as lipid nanoparticles, which enhance their stability and delivery to target cells.
Table Global Oligonucleotide Therapeutics Market Size by Type in 2024
Type | Market Size (M USD) 2024 |
Antisense Oligonucleotide | 3470.41 |
Small Interfering RNA | 1869.88 |
5 Global Oligonucleotide Therapeutics Market Size by Application in 2024
Neuromuscular diseases represent a significant area of focus for oligonucleotide therapeutics. In 2024, the market size for oligonucleotide therapeutics in treating neuromuscular diseases is estimated to be 1871.99 M USD. The growing prevalence of neuromuscular disorders such as Duchenne muscular dystrophy (DMD) and spinal muscular atrophy (SMA) has spurred research and development in this area. For example, antisense oligonucleotides have shown promise in modifying the splicing process of dystrophin pre – mRNA in DMD patients, aiming to restore the production of functional dystrophin protein.
The market size for oligonucleotide therapeutics in treating ATTR in 2024 is projected to be 955.70 M USD. ATTR is a rare and progressive disease caused by misfolding and aggregation of transthyretin protein. Oligonucleotide therapies, such as RNA interference (RNAi) – based drugs, have been developed to target the production of mutant or wild – type transthyretin. These therapies work by silencing the gene that codes for transthyretin, thereby reducing the levels of misfolded protein in the body.
For hepatic VOD, the market size of oligonucleotide therapeutics in 2024 is estimated at 288.43 M USD. Hepatic VOD is a serious complication often associated with hematopoietic stem cell transplantation. Oligonucleotide therapies are being explored to target the underlying molecular mechanisms involved in the development of hepatic VOD, such as inflammation and endothelial cell damage.
Table Global Oligonucleotide Therapeutics Market Size by Application in 2024
Application | Market Size (M USD) 2024 |
Neuromuscular Diseases | 1871.99 |
ATTR | 955.70 |
Hepatic VOD | 288.43 |
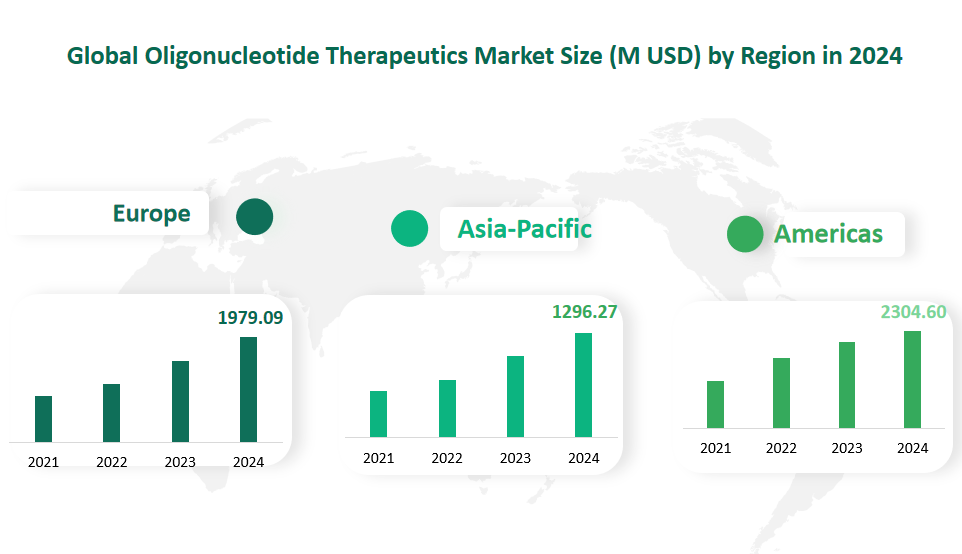
6 Global Oligonucleotide Therapeutics Market Size by Region in 2024
In 2024, the oligonucleotide therapeutics market in the Americas is estimated to be 2304.60 M USD. The United States, in particular, is a dominant force in this region. The Americas benefit from a well – developed healthcare infrastructure, significant investment in research and development, and a large patient population. Pharmaceutical and biotechnology companies in the region, such as Ionis Pharmaceuticals and Alnylam Pharmaceuticals, are at the forefront of oligonucleotide therapeutics research. The presence of major academic research institutions and government – funded research programs also contributes to the growth of the market.
The European oligonucleotide therapeutics market is projected to be 1979.09 M USD in 2024. Europe has a strong base of pharmaceutical and biotech companies, along with a highly educated workforce in the life sciences. Countries like the United Kingdom, Germany, and France are leading the way in research and development. The European Union has also implemented regulatory frameworks that support the development and approval of novel therapies, including oligonucleotide therapeutics. This has encouraged companies to invest in the region. For instance, the UK government has committed significant funds to healthcare research and manufacturing, which has facilitated the development of oligonucleotide – based drugs.
The Asia – Pacific region has seen rapid growth in the oligonucleotide therapeutics market, with an estimated market size of 1296.27 M USD in 2024. The region is home to a large and growing population, which presents a significant patient pool. Countries such as China, Japan, and South Korea are making substantial investments in biotechnology and pharmaceutical research. Japan, for example, has a well – established pharmaceutical industry and is actively involved in the development of oligonucleotide drugs. In China, the government has been promoting the development of the biotech industry, providing incentives for companies to invest in research and development.
Figure Global Oligonucleotide Therapeutics Market Size by Region in 2024
7 Major Players in Global Oligonucleotide Therapeutics Market
7.1 Alnylam Pharmaceuticals
Company Profile: Alnylam Pharmaceuticals, founded in 2002 and headquartered in Cambridge, Massachusetts, USA, is a trailblazing biopharmaceutical company. It has around 2,100 employees as of relevant data. The company is publicly traded on NASDAQ under the ticker symbol ALNY. Alnylam is committed to revolutionizing medicine through the development of RNA interference (RNAi) therapeutics. This innovative approach allows the company to target and silence specific genes that produce disease – causing proteins, making it a frontrunner in the genetic medicine field.
Business Overview: Alnylam’s business model is centered around discovering, developing, and commercializing RNAi – based drugs. It focuses on multiple therapeutic areas, including rare genetic diseases, cardio – metabolic diseases, hepatic infectious diseases, and central nervous system (CNS) or ocular diseases. The company has a diverse ownership structure, with major institutional investors such as BlackRock, Vanguard Group, and State Street Global Advisors, indicating its attractiveness in the biotech market.
Product Offered: The company has several marketed RNAi therapies. Onpattro is used for the treatment of the polyneuropathy of hereditary transthyretin – mediated amyloidosis (hATTR amyloidosis). Givlaari is indicated for acute hepatic porphyria, Amvuttra for the treatment of ATTR amyloidosis with cardiomyopathy, and Oxlumo for primary hyperoxaluria type 1 (PH1). In terms of its pipeline, it has numerous investigational RNAi drugs.
Financial Performance: In the most recent year, Alnylam Pharmaceuticals reported a revenue of 922 M USD.
7.2 Biogen
Company Profile: Biogen is a global biotechnology company with a long – standing reputation in the pharmaceutical industry. It was founded in 1978 and has its headquarters in Cambridge, Massachusetts. Biogen has a large global workforce and is publicly traded on NASDAQ under the ticker symbol BIIB. The company has been at the forefront of developing treatments for neurodegenerative diseases, but it also has a presence in other therapeutic areas.
Business Overview: Biogen’s business model encompasses research, development, manufacturing, and commercialization of biologic drugs. It has a broad portfolio of products and a strong focus on neuroscience, including diseases such as multiple sclerosis (MS), Alzheimer’s disease, and spinal muscular atrophy (SMA).
Product Offered: In the oligonucleotide therapeutics space, Biogen has contributed to the development of Spinraza (nusinersen) for the treatment of spinal muscular atrophy. Spinraza is an antisense oligonucleotide that has been a game – changer in the treatment of SMA, significantly improving the lives of patients. Besides this, Biogen has a range of products for multiple sclerosis, such as Tysabri (natalizumab), which is used to treat relapsing forms of MS.
Financial Performance: In the most recent year, Biogen reported a revenue of approximately 1.6 billion.
7.3 Sarepta Therapeutics
Company Profile: Sarepta Therapeutics is a biopharmaceutical company primarily focused on developing treatments for rare genetic diseases, especially those related to neuromuscular disorders. It was founded in 1980 and is headquartered in Cambridge, Massachusetts. The company has a dedicated workforce committed to addressing unmet medical needs in the rare disease space.
Business Overview: Sarepta’s business revolves around research, development, and commercialization of therapies for genetic disorders. It has a strong focus on muscular dystrophies, with a particular emphasis on Duchenne muscular dystrophy (DMD). The company’s approach involves using innovative gene – based therapies, such as exon – skipping technologies, to modify the expression of genes involved in these diseases.
Product Offered: The company’s most well – known product is Exondys 51 (eteplirsen), which is approved for the treatment of Duchenne muscular dystrophy in patients with a confirmed mutation amenable to exon 51 skipping. This was a significant milestone as it was the first FDA – approved drug using exon – skipping technology for DMD. Sarepta also has an extensive pipeline of investigational products.
Financial Performance: In the most recent year, Sarepta Therapeutics reported a revenue of approximately 708 million USD.

