1 Global Ranibizumab Market Outlook
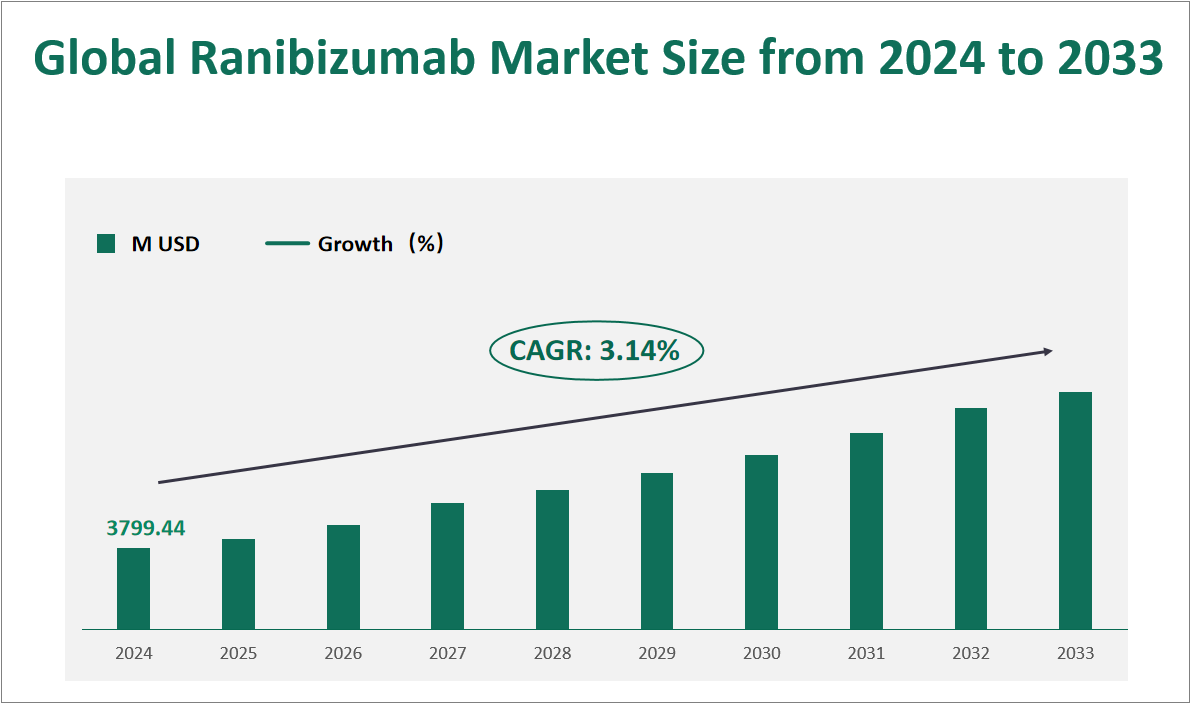
The global Ranibizumab market is projected to exhibit substantial growth in the coming years, with a CAGR of 3.14% from 2024 to 2033, reaching a total market size of $3799.44 million USD in 2024. Ranibizumab is designed to inhibit vascular endothelial growth factor-A (VEGF-A), a protein that plays a crucial role in the formation of new blood vessels. By targeting VEGF-A, ranibizumab helps reduce the leakage and growth of abnormal blood vessels in the eye, thereby alleviating symptoms associated with these conditions. The drug is available in two primary forms: single-use prefilled syringes and single-use glass vials. The prefilled syringes offer convenience and reduce the risk of contamination, making them increasingly popular among healthcare providers.
Figure Global Ranibizumab Market Size and Growth Rate (2024-2033)
2 Ranibizumab Market Growth Drivers and Constraints
Driving Factors
Increasing Prevalence of Eye Diseases: The rising incidence of age-related macular degeneration, diabetic retinopathy, and other retinal disorders is a significant driver of the ranibizumab market. As the global population ages and diabetes becomes more prevalent, the demand for effective treatments like ranibizumab is expected to grow.
Advancements in Ophthalmology: Technological advancements in ophthalmic diagnostics and treatment methods have improved the early detection and management of eye diseases. This has led to a higher demand for ranibizumab, as it is a proven treatment for several severe eye conditions.
Growing Awareness and Accessibility: Increased awareness about eye health and the availability of ranibizumab in more countries have expanded its market reach. Governments and healthcare organizations are also promoting regular eye check-ups, which further boosts the demand for effective treatments.
Limiting Factors
High Cost of Treatment: The cost of ranibizumab is relatively high, which can be a barrier for some patients, especially in regions with limited healthcare coverage. This financial burden can limit the accessibility of the drug to those who need it most.
Side Effects and Risks: Like any medical treatment, ranibizumab carries certain risks and side effects, such as increased intraocular pressure and retinal detachment. These risks can deter some patients and healthcare providers from using the drug.
Regulatory Hurdles: The stringent regulatory environment for pharmaceuticals can slow down the approval and availability of ranibizumab in some regions. Delays in approval can impact the market growth and patient access to the drug.
3 Ranibizumab Market Innovations and M&A Activities
Technology Innovation
In recent years, significant advancements in drug delivery systems and biotechnology have enhanced the efficacy and safety of ranibizumab. For instance, the development of single-use prefilled syringes has simplified the administration process, reducing the risk of contamination and improving patient outcomes. Additionally, ongoing research into personalized medicine and genetic markers is helping to tailor treatments more effectively to individual patients, further enhancing the drug’s market potential.
Corporate Mergers and Acquisitions
The ranibizumab market has seen several key mergers and acquisitions that have shaped its current landscape. For example, Novartis and Genentech, the primary manufacturers of ranibizumab, have collaborated closely to develop and market the drug globally. This partnership has allowed them to leverage each other’s strengths, expanding their market reach and enhancing their competitive position.
Moreover, the entry of generic versions of ranibizumab, such as Formycon’s FYB201, has introduced new dynamics into the market. These generic versions offer cost-effective alternatives, potentially increasing the overall accessibility of the drug while also intensifying competition among manufacturers.
In conclusion, the global ranibizumab market is poised for continued growth, driven by increasing demand for effective eye disease treatments and advancements in drug delivery technology. However, challenges such as high costs and regulatory hurdles need to be addressed to ensure that the benefits of this life-changing drug are accessible to all who need it.
4 Global Ranibizumab Market Analysis by Type
In 2024, the global ranibizumab market is forecasted to reach a total sales volume of 1,285,920 grams. The market is segmented by types, with single-use prefilled syringe accounting for 230,190 grams and single-use glass vial making up 1,055,730 grams. This translates to market shares of 17.90% for single-use prefilled syringe and 82.10% for single-use glass vial.
Table Global Ranibizumab Sales and Share by Type in 2024
Type | Sales in 2024 (K g) | Market Share in 2024 (%) |
|---|---|---|
Single-use Prefilled Syringe | 230.19 | 17.90% |
Single-use Glass Vial | 1055.73 | 82.10% |
5 Global Ranibizumab Market Analysis by Application
In 2024, the global ranibizumab market is forecasted to reach a total sales volume of 1,285,920 grams. The market is segmented by applications as follows:
wAMD (Wet Age-Related Macular Degeneration): Sales are projected to be 557,730 grams, accounting for 43.37% of the total market share.
Diabetic Retinopathy (DR): Sales are expected to reach 167,270 grams, representing 13.01% of the market share.
Diabetic Macular Edema (DME): Sales are forecasted at 207,050 grams, holding 16.10% of the market share.
mCNV (Myopic Choroidal Neovascularization): Sales are anticipated to be 109,390 grams, making up 8.51% of the market share.
RVO (Retinal Vein Occlusion): Sales are projected to be 244,480 grams, accounting for 19.01% of the market share.
These forecasts reflect the continued demand for ranibizumab across various applications, with wAMD remaining the largest segment.
Table Global Ranibizumab Sales and Share by Application in 2024
Application | Sales in 2024 (g) | Market Share in 2024 (%) |
|---|---|---|
wAMD | 557.73 | 43.37% |
Diabetic Retinopathy (DR) | 167.27 | 13.01% |
Diabetic Macular Edema (DME) | 207.05 | 16.10% |
mCNV | 109.39 | 8.51% |
RVO | 244.48 | 19.01% |
6 Global Ranibizumab Market Analysis by Region
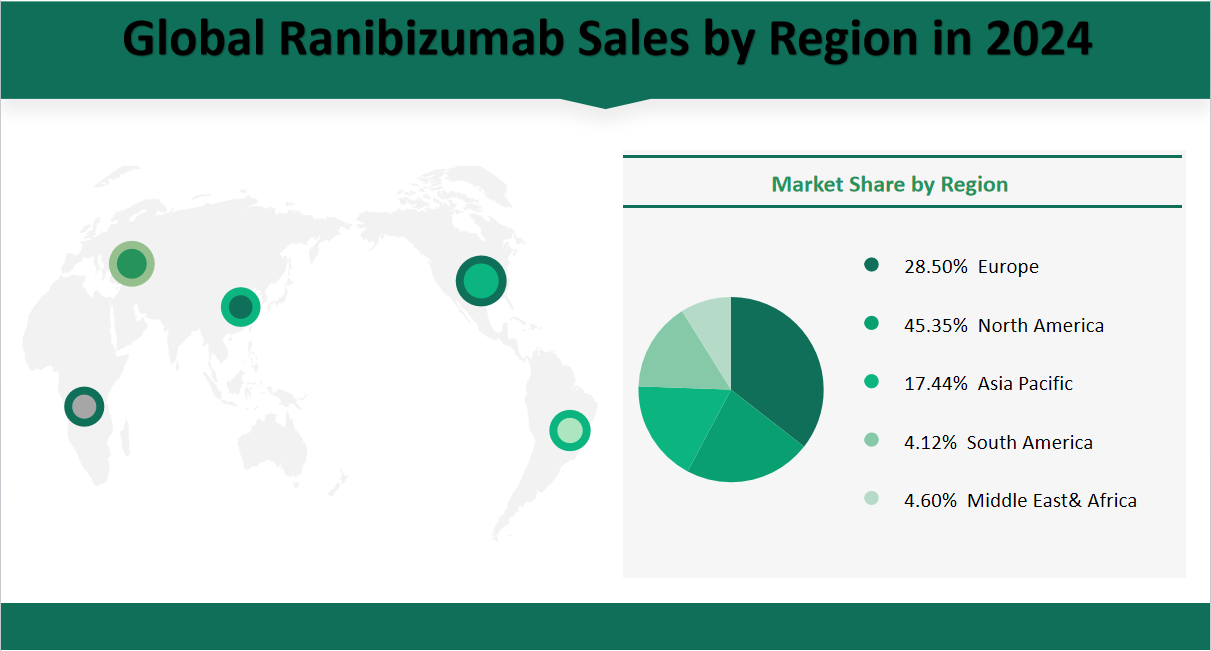
In 2024, the global ranibizumab market is forecasted to reach a total sales volume of 2,944.30 thousand vials. The market is segmented by regions as follows:
North America: Sales are projected to be 1,335.20 thousand vials, accounting for 45.35% of the total market share.
Europe: Sales are expected to reach 839.00 thousand vials, representing 28.50% of the market share.
Asia-Pacific: Sales are forecasted at 513.50 thousand vials, holding 17.44% of the market share.
South America: Sales are anticipated to be 121.20 thousand vials, making up 4.12% of the market share.
Middle East & Africa: Sales are projected to be 135.30 thousand vials, accounting for 4.60% of the market share.
These forecasts highlight the significant contributions of North America and Europe to the global ranibizumab market, with North America maintaining the largest market share.
Figure Global Ranibizumab Market Share by Region in 2024
7 Top 3 Companies of Global Ranibizumab Market
7.1 Novartis
Company Introduction and Business Overview:
Novartis is a leading global pharmaceutical company with a rich history dating back to its founding in 1996. Headquartered in Basel, Switzerland, Novartis operates in various segments, including pharmaceuticals, consumer health, and generics. The company is known for its commitment to innovation and its focus on improving health outcomes through cutting-edge research and development.
Novartis has a strong presence in the eye care segment, with a particular emphasis on the development and commercialization of ranibizumab. The company’s extensive research capabilities and global distribution network have enabled it to maintain a significant market share in the ranibizumab market.
Products Offered:
Novartis offers a range of products under the brand name Lucentis®, which includes:
Lucentis® Injection (Ranibizumab Injection): This product is indicated for the treatment of various eye conditions, including wet age-related macular degeneration (wAMD), diabetic macular edema (DME), and diabetic retinopathy (DR). It is available in both single-use prefilled syringes and single-use glass vials.
Lucentis® for Retinal Vein Occlusion (RVO): This formulation is specifically designed to treat macular edema following retinal vein occlusion.
Sales Revenue in the Latest Year:
Novartis reported a significant revenue from its ranibizumab products. The company’s sales figures for ranibizumab were as follows:
Sales (K Vials): 1,254.10 thousand vials
Revenue (M USD): $1,845 million
These figures highlight Novartis’s strong market position and its ability to meet the growing demand for ranibizumab treatments. The company’s robust sales performance in 2019 underscores its commitment to providing effective solutions for eye diseases and maintaining its leadership in the market.
7.2 Genentech
Company Introduction and Business Overview:
Genentech, a member of the Roche Group, is a biotechnology company with a strong focus on innovation and research. Established in 1976, Genentech is headquartered in South San Francisco, California, and is known for its pioneering work in the development of biopharmaceuticals. Genentech’s commitment to scientific excellence and patient care has made it a leader in the biotechnology industry.
Genentech’s collaboration with Novartis on the development and commercialization of ranibizumab has been instrumental in its success in the market. The company’s expertise in biotechnology and its extensive research capabilities have contributed significantly to the advancement of ranibizumab treatments.
Products Offered:
Genentech offers ranibizumab under the brand name Lucentis®, with the following formulations:
Lucentis® Injection (Ranibizumab Injection): This product is indicated for the treatment of wet age-related macular degeneration (wAMD), diabetic macular edema (DME), and diabetic retinopathy (DR). It is available in both single-use prefilled syringes and single-use glass vials.
Lucentis® for Retinal Vein Occlusion (RVO): This formulation is designed to treat macular edema following retinal vein occlusion.
Sales Revenue in the Latest Year:
Genentech reported robust sales figures for its ranibizumab products. The company’s sales data for ranibizumab were as follows:
Sales (K Vials): 1,008.90 thousand vials
Revenue (M USD): $1,536 million
These figures demonstrate Genentech’s strong market presence and its ability to meet the increasing demand for ranibizumab treatments. The company’s significant sales performance in 2019 reflects its commitment to innovation and its focus on improving patient outcomes.

