1 Global Sickle Cell Disease Diagnosis Market Insight Analysis
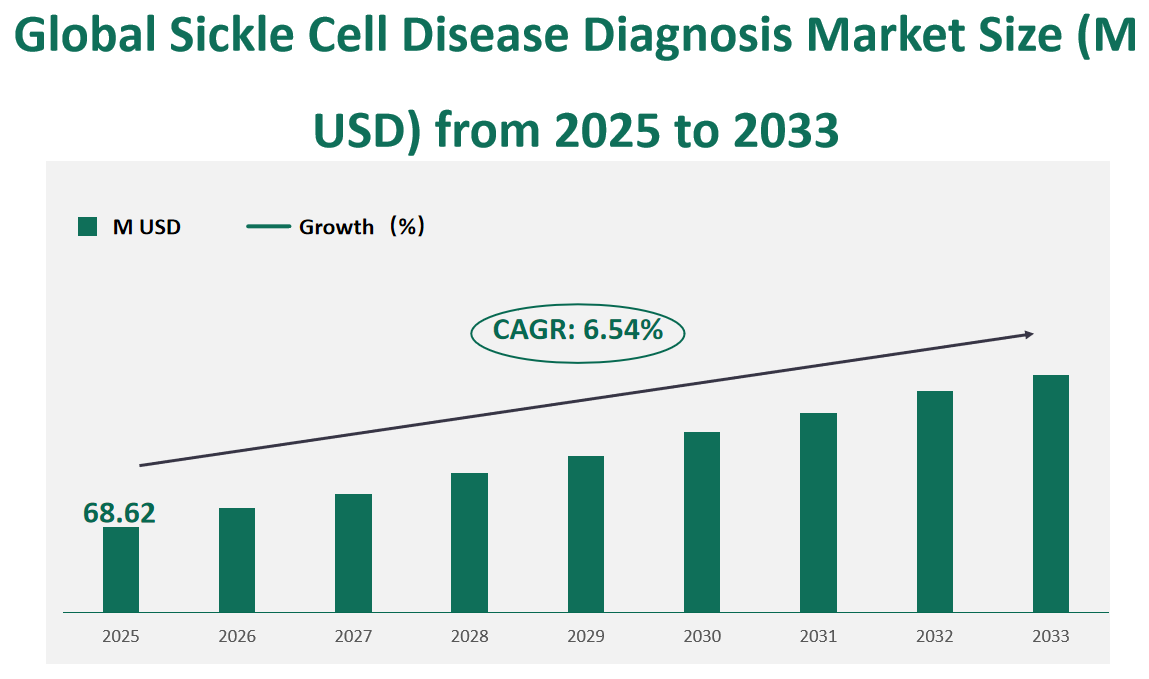
The global Sickle Cell Disease Diagnosis market will be valued at USD 68.62 million in 2025, with an estimated CAGR of 6.54% from 2025 to 2033.
Sickle Cell Disease Diagnosis refers to the medical procedures and tests used to identify the presence of sickle cell disease, a genetic disorder characterized by abnormal red blood cells. These tests are crucial for early detection and management of the disease, which can significantly improve patient outcomes. The market for SCD diagnosis includes various types of diagnostic tests, such as those that can distinguish between sickle cell disease and sickle cell trait, as well as those that cannot.
Figure Global Sickle Cell Disease Diagnosis Market Size (M USD) and CAGR (2025-2033)
2 Sickle Cell Disease Diagnosis Market Growth Drivers and Restraints
Disease burden and growing demand for testing
Sickle cell disease (SCD) is a common genetic blood disease in the world, especially in Africa, the Middle East and parts of Latin America. According to reports, about 300,000 newborns in Africa suffer from SCD each year, and 80%-90% of cases are concentrated there. The high neonatal mortality rate (50%-70% of SCD newborns in Africa die) has forced local governments and international organizations to increase screening efforts.
For example, the United States has passed legislation to enforce newborn screening, pushing the North American market to account for nearly half of the global market share (47.59% in 2019). In addition, although the demand for active testing for genetic risks by adult carriers (such as premarital testing) accounts for a low proportion (adult applications accounted for 5.65% in 2019), there is growth potential in this area as health awareness increases.
Economic development and policy support
Africa’s economic rise: The establishment of the African Continental Free Trade Area and the development of small and medium-sized enterprises have driven economic growth (Africa’s economic growth rate was 3.5% in 2018), improving medical payment capabilities. Although the overall situation is still underdeveloped, some countries have increased their investment in public health, driving the expansion of the SCD diagnostic market.
Policy and legislation: In 2018, the United States passed the Sickle Cell Disease Research and Prevention Act, authorizing funds to support the development of detection technology and monitoring projects; many European countries have included SCD in the scope of key genetic disease monitoring, and high-incidence countries such as France and the United Kingdom have promoted the popularization of testing. These policies directly stimulated market demand.
Technological progress and market competition
Detection technology has upgraded from “unable to distinguish between disease and carrier status” to “distinguishable”, driving the increase in the proportion of high-value-added products (in 2019, “distinguishable” types accounted for 85.15% of revenue share). Leading companies such as Fisher Scientific and Atlas Medical have consolidated their market position by developing high-precision test kits (such as Fisher’s SickleHeme™ test kit), while emerging companies (such as Silver Lake Research) have launched low-cost rapid testing products, targeting emerging markets such as Africa, and promoting technology accessibility.
Increased investment in global medical resources
International organizations such as the World Health Organization (WHO) have called for strengthening SCD prevention and control and promoting the introduction of screening programs in low- and middle-income countries. For example, although the Middle East and Africa market is currently small (accounting for 17.97% in 2019), economic growth and improved medical infrastructure have made it one of the fastest growing regions (expected to reach 18.85% in 2025).
Lack of awareness and low penetration of testing
In Africa and South Asia, where SCD is prevalent, the public’s awareness of the disease is limited, and many adults ignore the risk of carrying it because they are asymptomatic, resulting in a low pre-marital testing rate. In addition, the lack of training for primary medical personnel makes it difficult to promote standardized testing, especially in rural areas, where missed detection and misdiagnosis are prominent.
Economic and infrastructure bottlenecks
The economies of regions such as Africa are backward, and government finances are limited, making it difficult to afford the equipment and reagent costs for large-scale screening. For example, per capita medical expenditures in some countries in sub-Saharan Africa are less than US$100 per year, and the high cost of high-precision test kits (such as distinguishable types) limits their popularization. At the same time, imperfect cold chain logistics affects the preservation of reagents, further restricting the accessibility of testing.
Technical limitations and market competition barriers
The existing detection technology has bottlenecks: low-cost rapid detection products (such as Arlington Scientific’s test kits) cannot distinguish between diseases and carriers, which may lead to misdiagnosis; and high-precision detection requires professional equipment, which is difficult to promote in resource-poor areas. In addition, the market is highly concentrated (the top three companies account for 72.4% of the market share), and new entrants face technical patent barriers and channel monopoly, especially in mature markets such as North America and Europe.
Changes in population structure and disease characteristics
The decline in the number of newborns in some regions (such as Japan and Europe) may inhibit the growth of newborn testing demand. In addition, the incidence of SCD in East Asia and other regions is extremely low (China accounts for only 3.99% of the market), resulting in limited market expansion space in these regions.
3 Technological Innovations in the Sickle Cell Disease Diagnosis Market
High-precision and portable testing technology
Differential testing technology dominates the market: Reagents that can distinguish SCD from carriers (HbS/S vs. HbS/A) have become mainstream, accounting for 85.15% of revenue in 2019 and are expected to rise to 86.14% in 2025. For example, Atlas Medical’s test kit uses centrifugation to distinguish traits, and BioMedomics’ Sickle SCAN® uses immunochromatography technology to simultaneously detect HbA, HbS, and HbC to improve accuracy.
The rise of point-of-care testing (POCT) technology: Silver Lake Research’s HemoTypeSC™ test strips do not require buffer and cold chain, are suitable for resource-limited areas, and have strong stability (can be stored at high temperatures), filling the demand gap in markets such as Africa.
Low-cost and high-throughput solutions
For price-sensitive markets such as Africa, companies develop simplified testing solutions. For example, Fisher Scientific launched a 250-test large-volume test kit to reduce the cost of a single test; Arlington Scientific’s non-differentiated test kit is widely used in primary care due to its low price (about US$299-737/test kit).
Digitalization and automation integration
Some companies are exploring the combination of testing and digital platforms, such as recording test results and tracking patient disease progression through mobile applications, but this field is still in its infancy and is mainly used in regions with a high degree of medical informatization such as Europe and the United States.
Monopoly of leading companies and market concentration
The market is highly concentrated. In 2019, the top five companies accounted for 79.35% of the market share, of which Fisher Scientific ranked first with 50.56%, followed by Atlas Medical (12.50%) and BioMedomics (9.33%). Leading companies consolidate their position through patent layout (such as Fisher’s SickleHeme™ patented technology) and global distribution networks (such as distributors such as VWR and Tin Hang Technology).
Expansion of multinational companies: In 2015, Roper Technology acquired Atlas Medical to integrate its global distribution channels; in 2016, Abbott acquired Alere to obtain point-of-care testing technology and enter the SCD diagnostic market.
Layout of emerging markets: Silver Lake Research focuses on Africa and cooperates with local institutions to promote low-cost test strips; although Chinese companies have a small market share, they participate in the competition through the agency model (such as the introduction of imported reagents by Peking Union Medical College Hospital).
4 Global Sickle Cell Disease Diagnosis Market Size by Type
The segment of tests that can distinguish between sickle cell disease and sickle cell trait is expected to dominate the market by 2025. According to the forecast, this segment will generate a revenue of approximately 59.10 million USD. This type of test is crucial for accurate diagnosis, as it can differentiate between the disease (homozygous state, HbSS) and the carrier state (heterozygous state, HbAS).
The ability to make this distinction is vital for genetic counseling and family planning, particularly in regions with high prevalence of SCD. The market for these tests is driven by the need for precise diagnosis to manage the disease effectively and to prevent unnecessary anxiety and interventions for carriers who do not have the disease.
The segment of tests that cannot distinguish between sickle cell disease and sickle cell trait is also expected to grow, albeit at a slower pace. By 2025, this segment is projected to generate a revenue of about 9.51 million USD. These tests are typically used for initial screening purposes or in settings where the full range of diagnostic capabilities is not required. While they may be less expensive and quicker to perform, they do not provide the detailed information that is necessary for certain clinical decisions. Despite this limitation, the demand for these tests is expected to increase due to the broader screening efforts in populations at risk.
Table Global Sickle Cell Disease Diagnosis Market Size by Type in 2025
Type | Market Size (M USD) 2025 |
|---|---|
Can distinguish | 59.10 |
Cannot distinguish | 9.51 |
5 Global Sickle Cell Disease Diagnosis Market Size by Application
Among these, the newborn application segment is the dominant force in the market. It is projected to have a market value of 65.49 million US dollars in 2025. This significant portion can be attributed to several factors. Firstly, sickle – cell disease is a genetic disorder, and early detection in newborns is crucial for timely intervention and management. Many countries, especially those with a high prevalence of SCD such as in Africa, have implemented mandatory newborn screening programs. For example, in the United States, legislation has been in place to ensure widespread newborn screening for SCD, which has significantly contributed to the growth of this segment.
The adult application segment, on the other hand, is expected to have a market value of 3.13 million US dollars in 2025. Although this is a much smaller share compared to the newborn segment, it still holds importance. Adult testing is mainly carried out for reasons such as genetic counseling, especially for individuals who are carriers or have a family history of SCD.
It also serves as a diagnostic tool for adults who may start showing symptoms later in life. However, the relatively lower market value can be explained by the fact that the prevalence of symptomatic SCD in adults is not as high as the need for universal newborn screening. Also, awareness about adult testing is not as widespread as that for newborns in many regions.
Table Global Sickle Cell Disease Diagnosis Market Size and Share by Application in 2025
Application | Market Size (M USD) 2025 |
|---|---|
Newborn | 65.49 |
Adult | 3.13 |
6 Global Sickle Cell Disease Diagnosis Market Size by Region
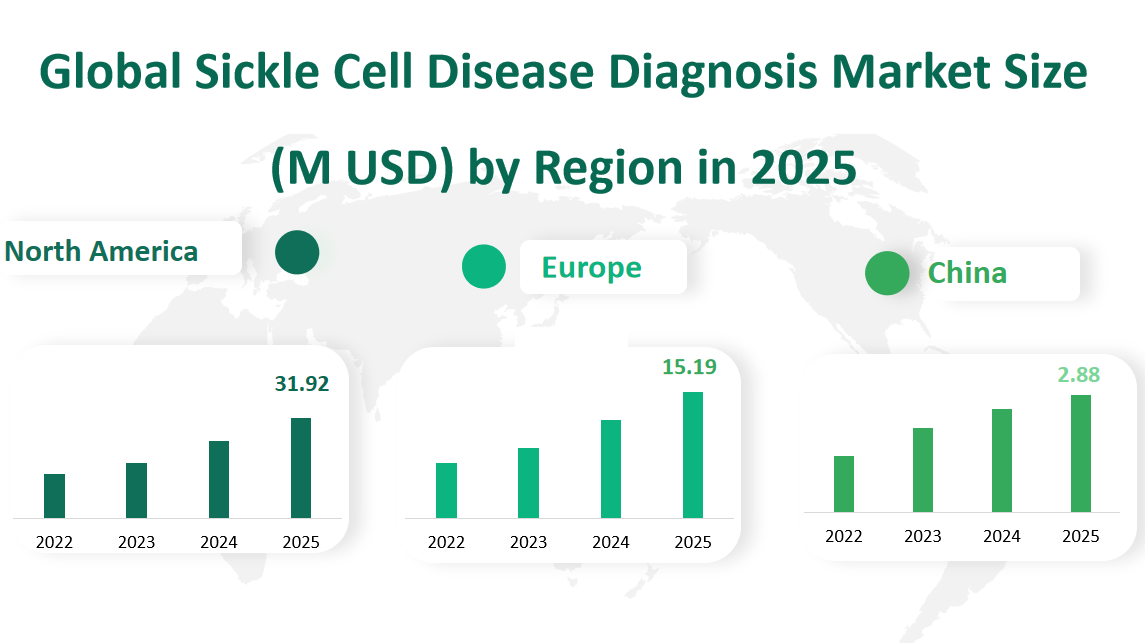
North America is expected to remain the largest market for SCD diagnosis, with a projected value of 31.92 million USD in 2025. The region’s dominance can be attributed to its well-established healthcare infrastructure, high awareness of genetic disorders, and significant investment in research and development. The United States, in particular, has a robust system for newborn screening, which includes SCD testing, contributing to the region’s large market size.
Europe is forecasted to be the second-largest market, with a value of 15.19 million USD in 2025. The European market is driven by the presence of key players in the diagnostic industry and the availability of advanced healthcare services. Additionally, the European Union’s efforts to standardize healthcare practices across member states are expected to boost the market further.
China is projected to have a market value of 2.88 million USD by 2025. Although SCD is less prevalent in China compared to other regions, the country’s growing healthcare sector and increasing focus on genetic testing are expected to drive market growth. The Chinese government’s initiatives to improve public health and the rising middle class’s access to advanced healthcare services are contributing factors.
Japan’s market is expected to reach 1.37 million USD by 2025. The country’s aging population and the government’s focus on early disease detection and prevention are key drivers. Japan’s high healthcare standards and the availability of advanced diagnostic technologies also support market growth.
The Middle East & Africa region is anticipated to have a market value of 12.93 million USD by 2025. The high prevalence of SCD in this region, particularly in African countries, is a significant driver. However, challenges such as limited healthcare infrastructure and economic constraints in some countries may hinder market growth. Efforts to improve healthcare access and awareness are crucial for the region’s market development.
India’s market is projected to reach 2.03 million USD by 2025. The country’s large population and the increasing prevalence of SCD, coupled with growing healthcare expenditure, are expected to drive market growth. Additionally, the Indian government’s initiatives to strengthen the healthcare sector and improve access to diagnostic services are likely to support market expansion.
South America is expected to have a market value of 0.91 million USD by 2025. The region’s market is driven by increasing awareness and the growing demand for advanced diagnostic services. However, economic and healthcare infrastructure challenges may affect market growth.
Figure Global Sickle Cell Disease Diagnosis Market Size (M USD) by Region in 2025
7 Global Sickle Cell Disease Diagnosis Market Analysis by Major Players
Fisher Scientific
Company Profile: Fisher Scientific, established in 1902, is a renowned name in the field of specialty chemicals and laboratory equipment. It operates as a part of Thermo Fisher Scientific and has a global presence, serving customers in various sectors including healthcare, pharmaceuticals, and academic research.
Business Overview: Fisher Scientific provides a comprehensive range of chemical products, including basic solutions, biochemicals, and bioreagents. The company’s worldwide distribution network ensures that its products reach a broad customer base, from small laboratories to large-scale research facilities. Fisher Scientific is committed to innovation and quality, which has helped it maintain a leading position in the market.
Product Offered: In the context of Sickle Cell Disease Diagnosis, Fisher Scientific offers the Great Lakes Diagnostics SickleHeme™ Sickle Cell Solubility Test Kits. These kits are designed for both low and high-volume users, with a shelf life of two years at room temperature. They include components for 90 days of coverage from one kit (for 12 and 100 tests) and a bottle buffer and powder reagent kit for high-volume users (250 tests). These products are crucial for accurate and efficient SCD diagnosis.
Atlas Medical
Company Profile: Atlas Medical, founded in 1996, is a global manufacturer and supplier of diagnostic reagents and kits. The company has a strong presence in over 80 countries, making it a significant player in the diagnostic market.
Business Overview: Atlas Medical specializes in providing diagnostic solutions that cater to a wide range of medical needs. Its products are known for their quality and reliability, which has helped the company establish strong relationships with healthcare providers worldwide. The company is dedicated to improving diagnostic capabilities and contributing to better patient outcomes.
Product Offered: Atlas Medical offers the Atlas Sickle Cell Kit, a qualitative solubility test for Sickle Cell Disease. This kit can be used in two ways: as a screening test to detect sickle haemoglobin (HbS) and as a centrifugation test to differentiate between the sickle cell trait (AS) and sickle cell anaemia (SS). The versatility of this kit makes it a valuable tool in the diagnosis and management of SCD.
BioMedomics Inc
Company Profile: BioMedomics Inc, established in 2006, is a leading provider of point-of-care diagnostic tests. The company focuses on developing innovative solutions for blood disorders and the identification of microorganisms.
Business Overview: BioMedomics operates globally, offering a range of diagnostic tests that are designed to be used in various healthcare settings, from hospitals to clinics. The company’s commitment to innovation and its focus on point-of-care testing have positioned it as a key player in the diagnostic industry.
Product Offered: BioMedomics offers the BioMedomics ™ Sickle SCAN ®, a multiplexed, qualitative, point-of-care immune test for rapid diagnostic assistance in sickle cell disease. This test includes three indicators that detect the presence of hemoglobin A, S, and C, allowing users to quickly distinguish between normal, carrier, and sickle cell disease samples. The Sickle SCAN ® is designed to be user-friendly and efficient, making it a valuable asset in the diagnosis of SCD.

