1 Global Biogeneric Drugs Market Insight Analysis
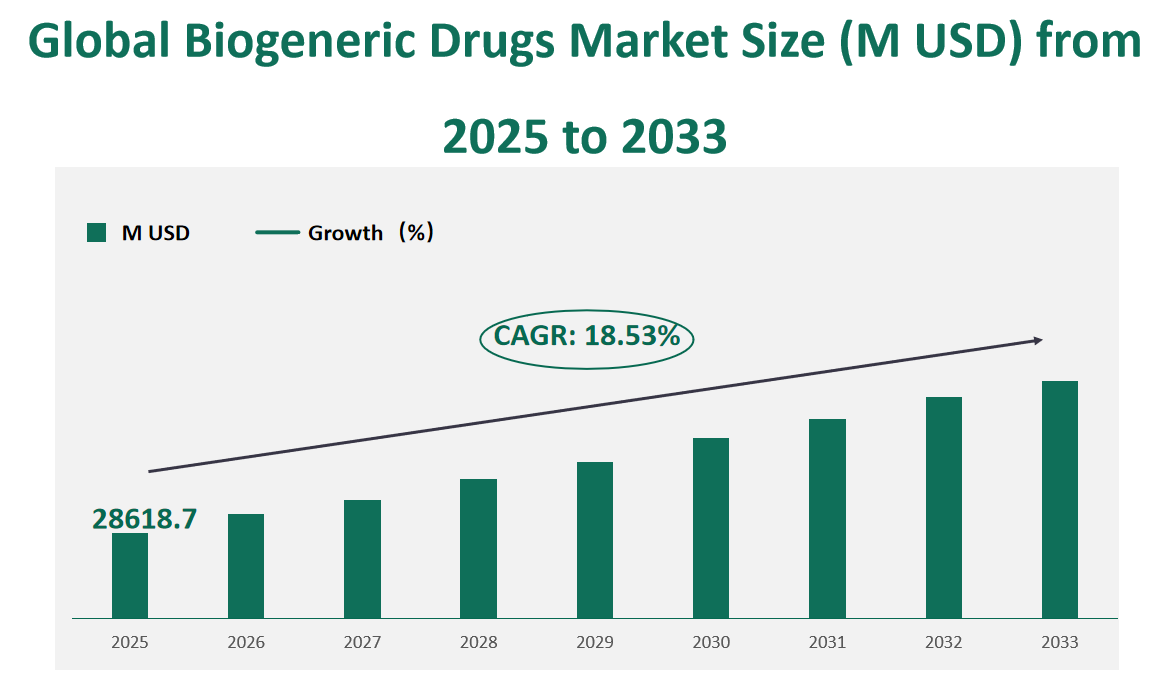
The global Biogeneric Drugs market is expected to be valued at USD 28618.7 million in 2025, with a CAGR of 18.53% from 2025 to 2033.
Biogeneric Drugs are biologics that closely resemble already approved biologic drugs (called reference products) and are made from natural sources (living systems such as yeast, bacteria, or animal cells). Biogeneric Drugs must have no clinically significant differences from the reference product, and be the same in terms of drug action mechanism, strength, dosage, safety and effectiveness, high-standard manufacturing, etc. Minor differences are allowed in clinically inactive ingredients. Biogeneric Drugs, which must be approved by the FDA, can improve patient access to care by increasing the number of drug options and reducing research and manufacturing costs.
Figure Global Biogeneric Drugs Market Size (M USD) and CAGR (2025-2033)
2 Biogeneric Drugs Market Growth Drivers and Restraints
Rising prevalence of chronic diseases and growing medical demand
The global incidence of chronic diseases such as cancer and diabetes continues to rise, driving the demand for biosimilars. For example, in 2020, the number of cancer deaths worldwide reached 10 million, and the number of diabetes patients exceeded 460 million. Biosimilars have become an important choice to alleviate the medical burden because they have the same efficacy as original drugs but lower costs (usually 30%-70% lower). Taking insulin as an example, its market size reached US$2.245 billion in 2023 and is expected to increase to US$5.447 billion in 2029 at a compound annual growth rate (CAGR) of 15.92%, mainly benefiting from the expansion of the base of diabetes patients.
Policy support and pressure on medical insurance cost control
In order to reduce medical expenses, governments of various countries actively promote biosimilars to replace original drugs. For example, the US FDA has accelerated the launch of biosimilars by simplifying the approval path (such as the Biologics Price Competition and Innovation Act), and approved the 40th biosimilar in 2022; Europe provides R&D subsidies through the “Priority Drug Program”; China has included biosimilars in the reimbursement catalog through medical insurance negotiations, and the market size will reach US$1.325 billion in 2023 (CAGR 26.1%), with significant policy dividends.
Technological progress and increased R&D investment
Companies have increased the research and development of high-end biosimilars and focused on complex molecules (such as monoclonal antibodies). In 2023, monoclonal antibodies accounted for 62.99% of the global biosimilar market, and it is expected to rise to 67.69% in 2029, with a CAGR of 19.96%. Leading companies such as Pfizer and Samsung Bio have shortened the R&D cycle and reduced costs by optimizing cell culture technology and analytical methods, while expanding into high-priced drug fields such as tumors and autoimmune diseases.
Emerging market growth potential is released
Asia-Pacific, Latin America and other regions have become growth engines due to their large population base and improved medical accessibility. With the advantage of low-cost production, India’s market size will reach US$1.07 billion in 2023 and is expected to reach US$3.96 billion in 2029 (CAGR 22.69%); China benefits from the rise of local companies such as Fosun Pharma, and its market size is expected to reach US$4.789 billion in 2029, with a CAGR of 26.5% from 2018 to 2029.
R&D complexity and high capital threshold
The production of biosimilars requires accurate replication of the molecular structure, production process and quality of the original drug, involving complex processes such as cell line development and purification process optimization. The R&D cost is usually hundreds of millions of dollars, and the failure rate is as high as 50%-75% (the failure rate of small molecule generics is only 5%). For example, the development of Amgen’s Humira biosimilar took more than 10 years and required multi-stage clinical comparative trials, making it difficult for small and medium-sized enterprises to enter.
Patent disputes and market exclusivity protection
Original drug companies extend their market exclusivity through patent cliff strategies, such as AbbVie’s Humira patent dispute, which delayed the launch of its biosimilars until 2023. Patent litigation in Europe, the United States and other places takes an average of 5-8 years, increasing the time cost and market risk of biosimilar companies.
Regulatory barriers and doctor-patient cognitive bias
Some countries have strict regulations on the interchangeability of biosimilars, such as the United States requiring additional clinical trials, while the European Union allows interchangeability but doctors prefer original drugs in prescriptions. In addition, patients have doubts about the safety of biosimilars. Surveys show that only 45% of American patients trust that biosimilars have the same efficacy as original drugs, and long-term education and promotion are needed.
Supply chain challenges and production concentration
Upstream raw materials (such as culture media and purification fillers) rely on imports, and production equipment (such as fermentation tanks) technology is monopolized by European and American companies. In 2023, the world’s top five manufacturers (Pfizer, Sandoz, and Teva) accounted for 44.91% of the market share, and small and medium-sized enterprises faced the dual pressures of unstable supply chains and insufficient economies of scale.
3 Technological Innovations in the Biogeneric Drugs Market
Complex biosimilars and novel formulation development
Monoclonal antibodies (mAbs): The market size in 2023 is $12.96 billion, accounting for 62.99%, focusing on tumors (such as bevacizumab biosimilars) and autoimmune diseases (such as adalimumab biosimilars). Samsung Biopharma’s Benepali (etanercept biosimilar) has covered 110 countries and extended half-life through Fc segment optimization.
Long-acting preparations and drug delivery systems: For example, growth hormone biosimilars have been changed from daily injections to once a week, and Novartis’ Omnitrope (growth hormone) has improved patient compliance through prefilled pens.
Emerging technology applications: mRNA technology extends from vaccines to biosimilars, such as Moderna and Teva’s collaboration to develop a biosimilar platform for mRNA delivery.
Production process innovation and green manufacturing
Continuous production technology: Sandoz introduced disposable bioreactors to shorten the production cycle from 30 days in traditional batch processes to 14 days, reducing costs by 30%.
Artificial intelligence (AI) assisted R&D: Pfizer uses AI to predict protein folding, accelerate antibody-antigen binding site analysis, and shorten the R&D cycle by 20%.
Sustainable packaging: Sanofi uses recyclable glass syringes to reduce plastic use by 40%, in line with the EU Green Deal.
Personalized and combined therapies
Companion diagnostics and biosimilars are jointly developed, such as Roche’s Herceptin biosimilars with HER2 detection kits to accurately screen patients who benefit.
Multi-target biosimilars (such as bispecific antibodies) have entered clinical trials, and Amgen’s AMG 162 (targeting RANKL) is used for osteoporosis, with sales exceeding US$1 billion in 2023.
Leading companies expand pipelines and market share through mergers and acquisitions
Pfizer: Acquired Arena Pharmaceuticals in 2022 to obtain the oral S1P receptor modulator etrasimod to expand the field of autoimmune diseases; in 2023, biosimilars revenue will be US$2.503 billion, with a global market share of 12.16%, ranking first.
Amgen: Acquired Horizon Therapeutics for $27.8 billion in 2022, included in rare disease drug portfolio, and its Humira biosimilar Amjevita achieved sales of $1.2 billion in its first year on the market.
Samsung Bio: Cooperated with Biogen to develop ophthalmic biosimilars, with revenue of $1.414 billion in 2023 and a market share of over 30% in South Korea.
Strategic integration of emerging market companies
Fosun Pharma: Introduced Kite Pharma’s CAR-T cell therapy Yescarta through its subsidiary Fosun Kite, which was launched in China in 2021 and became the first local CAR-T biologics. In 2023, biosimilars revenue was $848 million, a year-on-year increase of 31%.
Biocon Biologics: Acquired Viatris’ international biosimilar business in 2020, obtained insulin and oncology drug pipelines, with revenue of $470 million in 2023 and market share in India increased to 18%.
Cross-border cooperation and ecological construction
Alliance between pharmaceutical companies and technology companies: Novartis and Microsoft cooperated to develop a digital platform to track biosimilar efficacy data, covering 500,000 patients; Teva and Alvotech jointly developed Stelara biosimilars and used AI to optimize clinical trial design.
Regional market integration: Latin American companies such as Brazil EMS acquired Roche’s biosimilar business in Argentina to consolidate their market position in South America. The Latin American market size will be US$837 million in 2023, with a CAGR of 16.04%.
4 Global Biogeneric Drugs Market Size by Type
Insulin is a critical biogeneric drug type, essential for managing diabetes. By 2025, the insulin segment is projected to generate revenue of $3,028.1 million USD. This represents a significant portion of the overall market, with a market share of approximately 10.58%. The growth in this segment is driven by the increasing prevalence of diabetes worldwide, particularly in developed countries where lifestyle changes and aging populations have led to higher incidences of the disease. Additionally, advancements in insulin formulations and delivery systems have enhanced the efficacy and convenience of treatment, further boosting market demand.
Growth hormones are another important segment in the biogeneric drugs market, used primarily for treating growth disorders and other conditions related to hormone deficiencies. In 2025, the growth hormones segment is expected to achieve a revenue of $4,417.8 million USD, accounting for 15.44% of the total market share. The demand for growth hormones is driven by the increasing awareness of growth disorders and the availability of effective treatments. Moreover, the expanding pediatric patient population and advancements in recombinant DNA technology have contributed to the growth of this segment.
Monoclonal antibodies represent the largest segment in the biogeneric drugs market. By 2025, this segment is projected to generate a revenue of $18493.6 million USD, capturing 64.62% of the total market share. Monoclonal antibodies are versatile and are used in the treatment of various diseases, including cancer, autoimmune disorders, and infectious diseases. The growth in this segment is driven by the increasing incidence of chronic diseases, the development of new therapeutic applications, and the approval of multiple biosimilar products. Additionally, the cost-effectiveness of monoclonal antibody biosimilars compared to their branded counterparts has made them a preferred choice for healthcare providers and patients.
Table Global Biogeneric Drugs Market Size and Share by Type in 2025
Type | Market Size (M USD) 2025 | Market Share 2025 |
|---|---|---|
Insulin | 3028.1 | 10.58% |
Growth Hormones | 4417.8 | 15.44% |
Monoclonal Antibodies | 18493.6 | 64.62% |
Others | 2679.3 | 9.36% |
5 Global Biogeneric Drugs Market Size by Application
Hospitals were the dominant application area for biogeneric drugs in 2025, with a revenue of 21,217.7 million US dollars. This accounted for 74.14% of the total market revenue. Hospitals serve as the primary healthcare providers for complex and severe medical conditions, where biogeneric drugs play a crucial role. For example, in treating chronic diseases such as cancer, diabetes, and autoimmune disorders, hospitals administer a wide range of biogeneric drugs.
These medications are often more cost – effective compared to their originator counterparts, making them an attractive option for hospitals aiming to manage healthcare costs while maintaining high – quality patient care. The large revenue figure for hospitals in the biogeneric drugs market reflects the high volume of drug consumption in inpatient and outpatient settings, as well as the increasing prevalence of diseases that require long – term and intensive medical treatment.
Clinics generated a revenue of 6,476.5 million US dollars in 2025, holding a market share of 22.63%. Clinics are essential in providing primary and specialized outpatient care. Biogeneric drugs are increasingly being prescribed in clinics for various conditions, including routine chronic disease management, minor surgical procedures, and preventive healthcare. They offer an affordable alternative for patients seeking treatment outside of hospital settings.
As the demand for accessible and cost – efficient healthcare services grows, clinics are likely to continue playing a significant role in the distribution and use of biogeneric drugs. This segment is also expected to expand further as more patients opt for outpatient treatment options to avoid the high costs associated with hospital stays.
Research centers had a relatively smaller but still important share in the biogeneric drugs market in 2025. Their revenue stood at 924.5 million US dollars, accounting for 3.23% of the total market revenue. Research centers are at the forefront of clinical trials and research on biogeneric drugs. They are involved in evaluating the safety, efficacy, and quality of these drugs, which is crucial for their approval and subsequent market entry.
The revenue in this segment is generated from research grants, contracts with pharmaceutical companies, and government funding for drug development and testing. The role of research centers is indispensable in advancing the field of biogeneric drugs, ensuring that they meet the necessary regulatory standards and can provide real – world benefits to patients.
Table Global Biogeneric Drugs Market Size and Share by Application in 2025
Application | Market Size (M USD) 2025 | Market Share 2025 |
|---|---|---|
Hospital | 21217.7 | 74.14% |
Clinics | 6476.5 | 22.63% |
Research Centers | 924.5 | 3.23% |
6 Global Biogeneric Drugs Market Size by Region
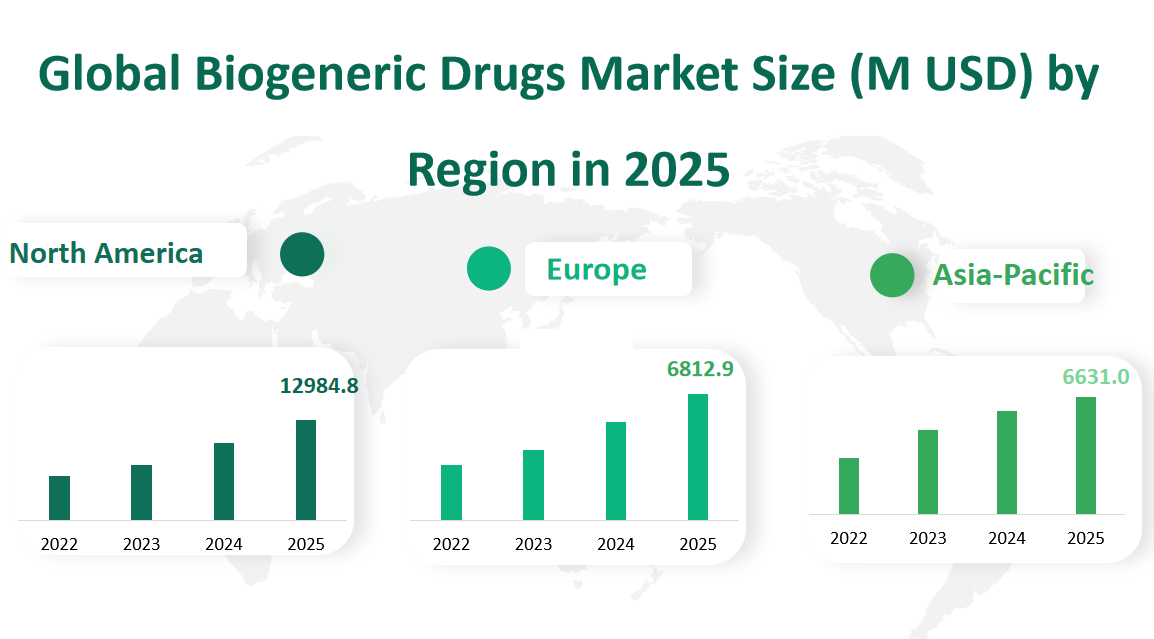
In 2025, North America led the global biogeneric drugs market in terms of revenue, which reached 12,984.8 million US dollars. This region has a well – established healthcare infrastructure, a high prevalence of chronic diseases such as cancer and diabetes, and a strong emphasis on cost – effective healthcare solutions. The United States, in particular, has been a major contributor to this revenue. The country’s regulatory framework, such as the Biologics Price Competition and Innovation Act, has facilitated the entry of biogeneric drugs into the market. Moreover, the high – spending power of consumers and the extensive insurance coverage for biopharmaceuticals have further boosted the market.
Europe followed with a market revenue of 6,812.9 million US dollars in 2025. The European biogeneric drugs market benefits from a mature pharmaceutical industry, with countries like Germany, the United Kingdom, and France playing key roles. The European Union’s regulatory environment promotes the development and approval of biogeneric drugs. Additionally, the growing pressure on healthcare systems to control costs has led to an increased adoption of biogeneric drugs. However, challenges such as patent disputes and differences in regulatory requirements across member states still exist.
The Asia – Pacific region recorded a market revenue of 6,631.0 million US dollars in 2025. This region has been experiencing rapid growth, driven by factors such as a large population base, increasing healthcare expenditure, and a growing middle – class with improved access to healthcare. China and India are the two major powerhouses in the Asia – Pacific biogeneric drugs market. India, known as the “pharmacy of the world,” has a strong manufacturing base and is a major exporter of biogeneric drugs. China, on the other hand, has been investing heavily in research and development, and local biopharmaceutical companies are making significant progress in developing high – quality biogeneric drugs.
Latin America’s biogeneric drugs market revenue in 2025 was 1,149.4 million US dollars. The region has been gradually increasing its share in the global market. The growth is mainly attributed to the improving economic conditions in some countries, increasing government support for healthcare, and a rising demand for affordable medications. Brazil, for example, has been actively promoting the use of biogeneric drugs through various policies and initiatives.
In 2025, the Middle East and Africa region had a market revenue of 1,040.5 million US dollars. This region faces unique challenges and opportunities. The increasing prevalence of non – communicable diseases, such as diabetes and cardiovascular diseases, has led to a growing demand for biogeneric drugs. However, issues such as limited healthcare infrastructure in some areas, low awareness about biogeneric drugs, and difficulties in accessing advanced medical technologies still pose challenges. Nevertheless, countries in the Middle East, with their relatively high – income levels and investment in healthcare, are driving the growth of the market in this region.
Figure Global Biogeneric Drugs Market Size (M USD) by Region in 2025
7 Global Biogeneric Drugs Market Analysis by Major Players
Pfizer
Company Profile:
Pfizer is one of the world’s leading biopharmaceutical companies, with a rich history dating back to 1849. Headquartered in New York, USA, Pfizer operates globally, serving patients in over 125 countries. The company is renowned for its innovative research and development capabilities, as well as its extensive portfolio of pharmaceutical products.
Business Overview:
Pfizer’s business spans the entire spectrum of healthcare, from research and development to manufacturing and commercialization. The company focuses on discovering and developing treatments for a wide range of diseases, including cardiovascular, metabolic, oncological, inflammatory, and rare diseases. Pfizer’s commitment to innovation and patient care has led to the development of numerous groundbreaking therapies and vaccines.
Product Offered:
Pfizer offers a diverse range of biogeneric drugs, including biosimilars such as Inflectra (infliximab) and Truxima (rituximab). These biosimilars are designed to provide cost-effective alternatives to existing biologic treatments, ensuring that more patients have access to life-changing therapies. Pfizer’s biosimilar portfolio is continuously expanding, with ongoing research and development efforts aimed at addressing unmet medical needs in various therapeutic areas.
Sandoz International
Company Profile:
Sandoz International, a division of Novartis, is a global leader in generic pharmaceuticals and biosimilars. Established in 1886, Sandoz has a long history of pioneering novel approaches to improve patient access to high-quality medicines. Headquartered in Basel, Switzerland, Sandoz operates in over 140 countries, making it one of the most widely recognized generic pharmaceutical companies globally.
Business Overview:
Sandoz’s business is centered around providing affordable, high-quality generic and biosimilar medicines to patients worldwide. The company invests significantly in research and development to enhance the lives of patients and liberate healthcare resources. Sandoz’s biosimilar portfolio includes eight marketed products across immunology, oncology, and endocrinology, with over 15 molecules in various stages of development. This commitment to innovation and accessibility has solidified Sandoz’s position as a leader in the biosimilar market.
Product Offered:
Sandoz offers a comprehensive range of biogeneric drugs, including biosimilars such as Zarxio (filgrastim) and Erelzi (etanercept). These products are designed to provide cost-effective alternatives to branded biologics, ensuring that patients have access to essential treatments. Sandoz’s biosimilar portfolio is continuously expanding, with ongoing efforts to develop new products and improve existing ones. The company’s focus on quality and innovation has led to the development of biosimilars that meet the highest regulatory standards, ensuring patient safety and efficacy.
Teva Pharmaceutical Industries
Company Profile:
Teva Pharmaceutical Industries, founded in 1901, is a leading global pharmaceutical company headquartered in Petah Tikva, Israel. Teva is renowned for its extensive portfolio of generic and specialty branded drugs, making it a major player in the global pharmaceutical market. The company’s commitment to innovation and patient care has led to the development of numerous groundbreaking therapies.
Business Overview:
Teva’s business encompasses the entire pharmaceutical value chain, from research and development to manufacturing and commercialization. The company focuses on providing affordable, high-quality medicines to patients worldwide. Teva’s portfolio includes a wide range of products, from generic drugs to biosimilars and specialty pharmaceuticals. The company’s strong market position and innovative approach have made it a leader in the generic pharmaceutical industry.
Product Offered:
Teva offers a diverse range of biogeneric drugs, including biosimilars such as Herzuma (trastuzumab) and Semglee (insulin glargine). These biosimilars are designed to provide cost-effective alternatives to branded biologics, ensuring that patients have access to essential treatments. Teva’s biosimilar portfolio is continuously expanding, with ongoing research and development efforts aimed at addressing unmet medical needs in various therapeutic areas. The company’s focus on innovation and quality has led to the development of biosimilars that meet the highest regulatory standards, ensuring patient safety and efficacy.

