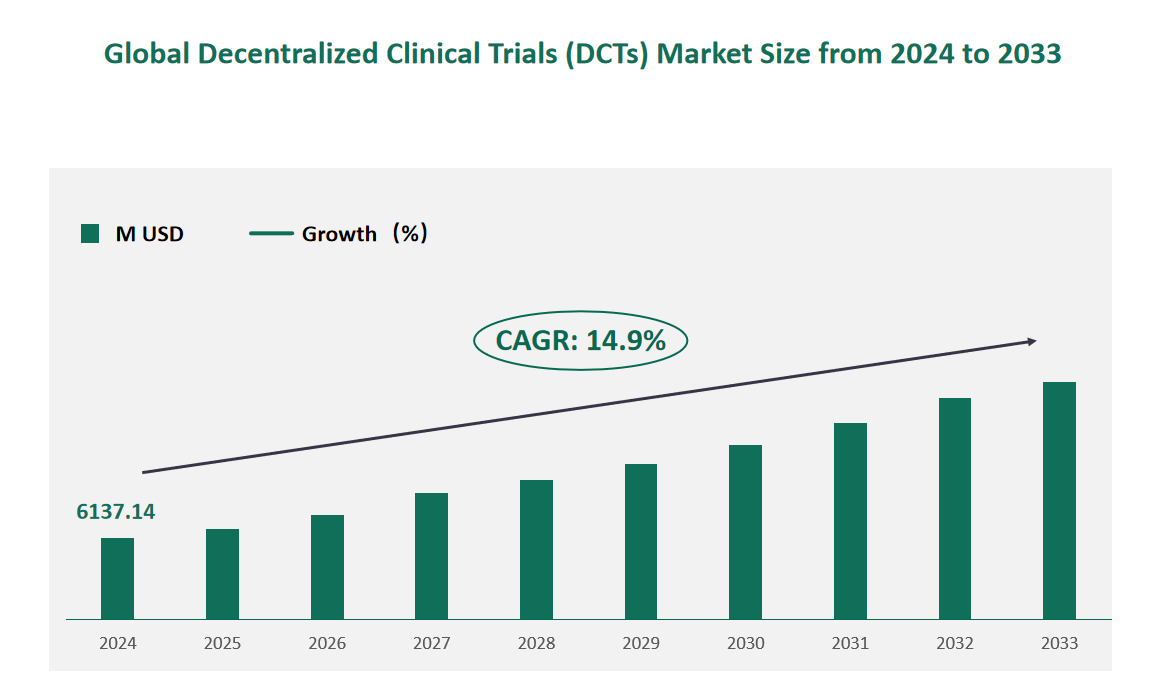
1 Global Decentralized Clinical Trials (DCTs) Market Size (Value) and CAGR (2024-2033)
In 2024, the global Decentralized Clinical Trials (DCTs) market was valued at USD 6137.14 million, with a CAGR of 14.9% from 2024 to 2033.
Decentralized Clinical Trials (DCTs) represent a paradigm shift in the way clinical research is conducted. Unlike traditional trials that rely heavily on centralized research sites, DCTs leverage telemedicine, mobile health technologies, and local healthcare providers to execute trials. This approach allows for greater flexibility, reduced logistical burdens, and enhanced patient accessibility. By utilizing digital tools such as electronic consent (eConsent), remote monitoring, and wearable devices, DCTs can significantly streamline the research process while maintaining high standards of data integrity and patient safety.
Figure Global Decentralized Clinical Trials (DCTs) Market Size (M USD) and CAGR 2024-2033
2 Decentralized Clinical Trials (DCTs) Market Drivers
One of the most significant drivers of the DCTs market is the rapid advancement in digital health technologies. The integration of telemedicine, wearable devices, electronic health records (EHRs), and mobile health applications has revolutionized the way clinical trials are conducted. These technologies enable real-time data collection, remote patient monitoring, and enhanced patient engagement, making trials more efficient and accessible. Additionally, the use of artificial intelligence (AI) and machine learning (ML) algorithms allows for better data analysis and predictive modeling, further enhancing the value of DCTs.
DCTs prioritize patient convenience and accessibility, addressing the limitations of traditional trials that often exclude patients due to geographical constraints or logistical challenges. By leveraging remote monitoring and telemedicine, DCTs can reach a broader and more diverse patient population, including those in rural or underserved areas. This not only improves patient recruitment and retention but also enhances the representativeness of trial results, leading to more reliable and applicable outcomes.
Regulatory bodies such as the FDA and EMA have shown increasing support for DCTs, recognizing their potential to accelerate drug development and improve patient outcomes. The establishment of the Decentralized Trial and Research Alliance (DTRA) further underscores the industry’s commitment to overcoming barriers and promoting the adoption of decentralized trials. This collaborative effort aims to standardize practices, address regulatory concerns, and provide guidance for stakeholders, thereby facilitating the growth of the DCTs market.
3 Decentralized Clinical Trials (DCTs) Market Restraints
While digital health technologies are a driving force for DCTs, they also present significant challenges. Ensuring the reliability and accuracy of data collected through remote monitoring devices and electronic health records is crucial for the validity of trial results. Additionally, data privacy and security concerns are paramount, especially given the sensitive nature of patient information. Compliance with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe adds an extra layer of complexity to the implementation of DCTs.
Regulatory frameworks for DCTs are still evolving, and there is a lack of standardized guidelines across different regions. This creates uncertainty for sponsors and researchers, who must navigate varying requirements and ensure compliance with local regulations. The validation of digital endpoints and the acceptance of remote data collection methods by regulatory bodies are ongoing challenges that need to be addressed to facilitate wider adoption of DCTs.
Implementing DCTs requires significant changes to traditional trial operations, including the integration of new technologies, training of staff, and coordination with multiple stakeholders. Ensuring seamless communication and data flow between patients, healthcare providers, and research teams can be complex and resource-intensive. Additionally, the reliance on remote monitoring and telemedicine may introduce variability in data quality and patient engagement, which can impact the reliability of trial outcomes.
4 Global Decentralized Clinical Trials (DCTs) Market Size and Share by Type in 2024
Interventional Trials are a core component of the DCTs market, focusing on evaluating the safety and efficacy of specific interventions, such as new drugs, medical devices, or therapeutic procedures. These trials involve participants receiving specific treatments according to a predefined protocol. In 2024, the market value for Interventional Trials is projected to be US$ 1,286.29 million. This segment is characterized by its direct impact on patient outcomes and its critical role in advancing medical treatments. The growth of this segment is driven by the increasing demand for new therapies and the need to streamline the drug development process.
Observational Trials represent the largest segment of the DCTs market, accounting for the majority of the market value. These trials involve observing participants in their natural settings without introducing any specific interventions. The goal is to gather data on the natural progression of diseases, the effectiveness of existing treatments, and the impact of various factors on health outcomes. In 2024, the market value for Observational Trials is projected to be US$ 4,712.19 million. This segment is crucial for understanding real-world data and generating insights that can inform future interventional studies and healthcare policies.
Expanded Access Trials are designed to provide patients with access to investigational drugs or treatments outside of traditional clinical trials. These trials are particularly important for patients with serious or life-threatening conditions who may not have other treatment options. In 2024, the market value for Expanded Access Trials is projected to be US$ 138.65 million. This segment highlights the ethical and humanitarian aspects of clinical research, ensuring that patients can benefit from new therapies while still contributing to the overall body of medical knowledge.
Table Global Decentralized Clinical Trials (DCTs) Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
Interventional Trials | 1286.29 | 20.96% |
Observational Trials | 4712.19 | 76.78% |
Expanded Access Trials | 138.65 | 2.26% |
5 Global Decentralized Clinical Trials (DCTs) Market Size and Share by Application in 2024
Oncology remains one of the most prominent applications for DCTs, driven by the increasing incidence of cancer worldwide and the need for more efficient clinical trials. In 2024, the market value for DCTs in oncology is projected to be US$ 1,649.75 million. This segment is crucial for developing new cancer therapies and improving patient outcomes through more accessible and patient-centric trial designs.
Cardiovascular diseases are another significant area where DCTs are increasingly being adopted. In 2024, the market value for DCTs in this application is projected to be US$ 958.42 million. The use of DCTs in cardiovascular research helps in gathering real-world data, improving patient recruitment, and reducing the burden on participants, thereby accelerating the development of new treatments.
Table Global Decentralized Clinical Trials (DCTs) Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
Oncology | 1649.75 | 26.88% |
Cardiovascular Disease | 958.42 | 15.62% |
Others | 3528.96 | 57.50% |
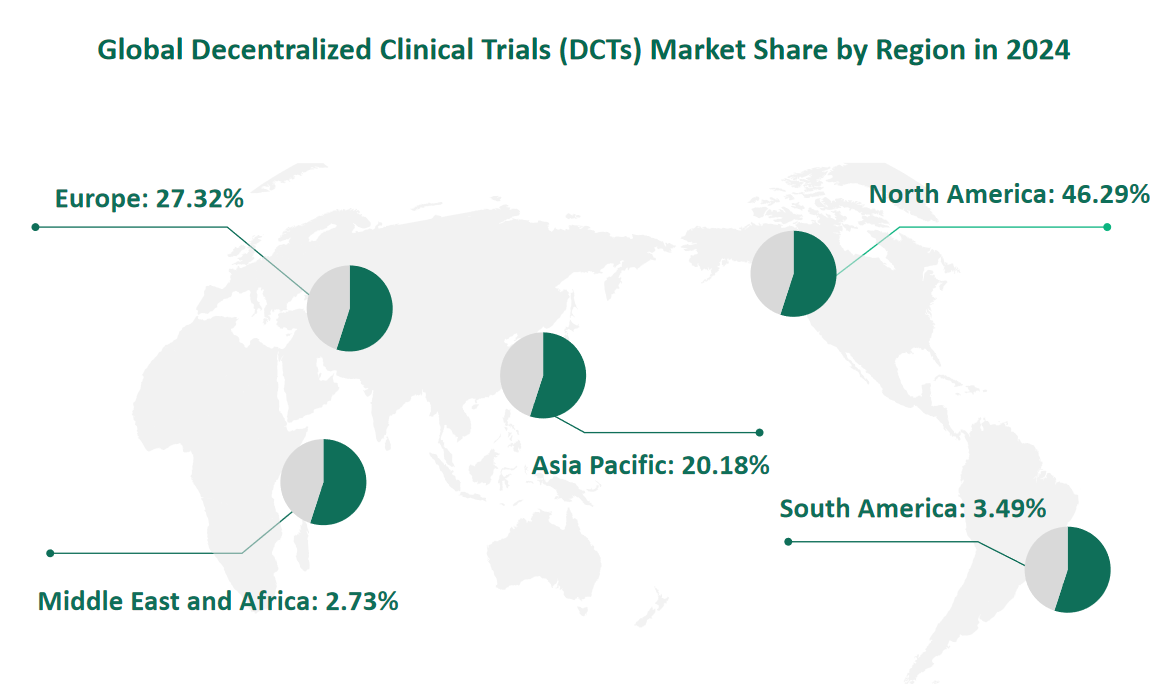
6 Global Decentralized Clinical Trials (DCTs) Market Size by Region in 2024
North America remains the largest market for DCTs, driven by technological advancements, regulatory support, and a high demand for efficient clinical research. In 2024, the market value for DCTs in North America is projected to be US$ 2,840.73 million. The United States, in particular, is a leader in adopting DCTs, supported by a robust digital health infrastructure and a favorable regulatory environment.
Europe is another significant market for DCTs, with a projected market value of US$ 1,676.78 million in 2024. The region benefits from strong regulatory frameworks and a growing emphasis on patient-centric research. Countries such as Germany and the United Kingdom are at the forefront of adopting DCTs, driven by government support and a focus on innovation in clinical trials.
The Asia-Pacific region is emerging as a fast-growing market for DCTs, driven by economic development, technological progress, and increasing downstream demand. In 2024, the market value for DCTs in the Asia-Pacific region is projected to be US$ 1,238.19 million. China and Japan are key markets in this region, with significant investments in digital health and clinical research infrastructure.
Figure Global Decentralized Clinical Trials (DCTs) Market Size by Region in 2024
7 Major Players in Global Decentralized Clinical Trials (DCTs) Market
7.1 IQVIA
Company Profile:
IQVIA is a global leader in healthcare data analytics and technology solutions. Established in 1982 and headquartered in the United States, IQVIA provides a wide range of services, including data science, clinical research, and healthcare consulting. The company is renowned for its ability to leverage advanced analytics and technology to drive healthcare forward.
Business Overview:
IQVIA offers comprehensive solutions for the healthcare industry, including clinical trial management, data analytics, and healthcare consulting. Their services are designed to help clients navigate the complexities of drug development and healthcare delivery. IQVIA’s Virtual Trials solution is a key component of their DCTs offerings, enabling sponsors to conduct trials remotely and reach diverse patient populations.
Product Introduction:
IQVIA’s Virtual Trials bring studies directly to patients, offering a flexible and patient-centric approach. Their platform, IQVIA Study Hub, is built on a highly secure, scalable SaaS model, allowing for seamless orchestration of all virtual or hybrid study activities. This solution is particularly valuable for sponsors looking to expand their geographic reach and improve patient engagement.
Recent Financial Data:
In the most recent year, IQVIA reported a revenue of US$ 537.61 million in the DCTs segment, with a gross margin of 40.74%.
7.2 PPD
Company Profile:
PPD, Inc. is a leading global contract research organization (CRO) with extensive experience in drug discovery, clinical development, and lifecycle management. Established in 1985 and headquartered in the United States, PPD serves pharmaceutical, biotechnology, medical device, and government organizations worldwide.
Business Overview:
PPD provides a comprehensive suite of services, including clinical research, laboratory services, and regulatory consulting. Their expertise in decentralized clinical trials is particularly notable, with a focus on leveraging digital technologies to improve patient engagement and trial efficiency. PPD’s digital trial solutions include electronic patient consent, telehealth, and hybrid trial models, enabling biopharmaceutical companies to advance their research programs.
Product Introduction:
PPD’s Decentralized Clinical Trials solution is designed to enhance patient engagement and retention by utilizing digital tools such as eConsent, telehealth, and remote monitoring. This approach not only improves the patient experience but also accelerates trial timelines and reduces logistical burdens.
Recent Financial Data:
In the most recent year, PPD reported a revenue of US$ 438.90 million in the DCTs segment, with a gross margin of 38.18%.
7.3 PRA Health Sciences
Company Profile:
PRA Health Sciences is a global healthcare intelligence partner, consistently ranked among the top CROs worldwide. Established in 1976 and headquartered in the United States, PRA provides comprehensive clinical development services, including data management, statistical analysis, and regulatory consulting.
Business Overview:
PRA Health Sciences offers a wide range of services for the healthcare industry, focusing on clinical trial management, medical writing, and drug development consulting. Their expertise in decentralized clinical trials is highlighted by their ability to combine medical informatics with advanced data analytics, enabling more efficient and patient-centric research. PRA’s Mobile Health platform is a key component of their DCTs offerings, allowing for remote patient engagement and data collection.
Product Introduction:
PRA’s Decentralized Clinical Trials solution emphasizes improved patient access and enrollment through the use of digital health technologies. Their platform includes eConsent, virtual visits, and wearable devices, enabling patients to participate in trials from the comfort of their homes. This approach not only enhances patient diversity but also accelerates trial timelines.
Recent Financial Data:
In the most recent year, PRA Health Sciences reported a revenue of US$ 309.83 million in the DCTs segment, with a gross margin of 40.30%.

