1 Global Pharmaceutical Aseptic Fill & Finish CMO Market Outlook
The global Pharmaceutical Aseptic Fill & Finish CMO market is projected to exhibit substantial growth in the coming years, with a CAGR of 8.06% from 2024 to 2033, reaching a total market size of $3830.35million USD in 2024. Pharmaceutical Aseptic Fill & Finish CMO refers to the process of transferring sterile drugs from a filling needle to a sterile container, typically a vial or pre-filled syringe, and completing the packaging process for distribution. This process is critical in the pharmaceutical industry, ensuring that drugs are safely and efficiently prepared for use. The market for these services has expanded significantly due to increasing demand for high-quality, sterile pharmaceutical products, particularly in the context of growing global health needs and the rise of biologic drugs.
Figure Global Pharmaceutical Aseptic Fill & Finish CMO Market Size and Growth Rate (2024-2033)
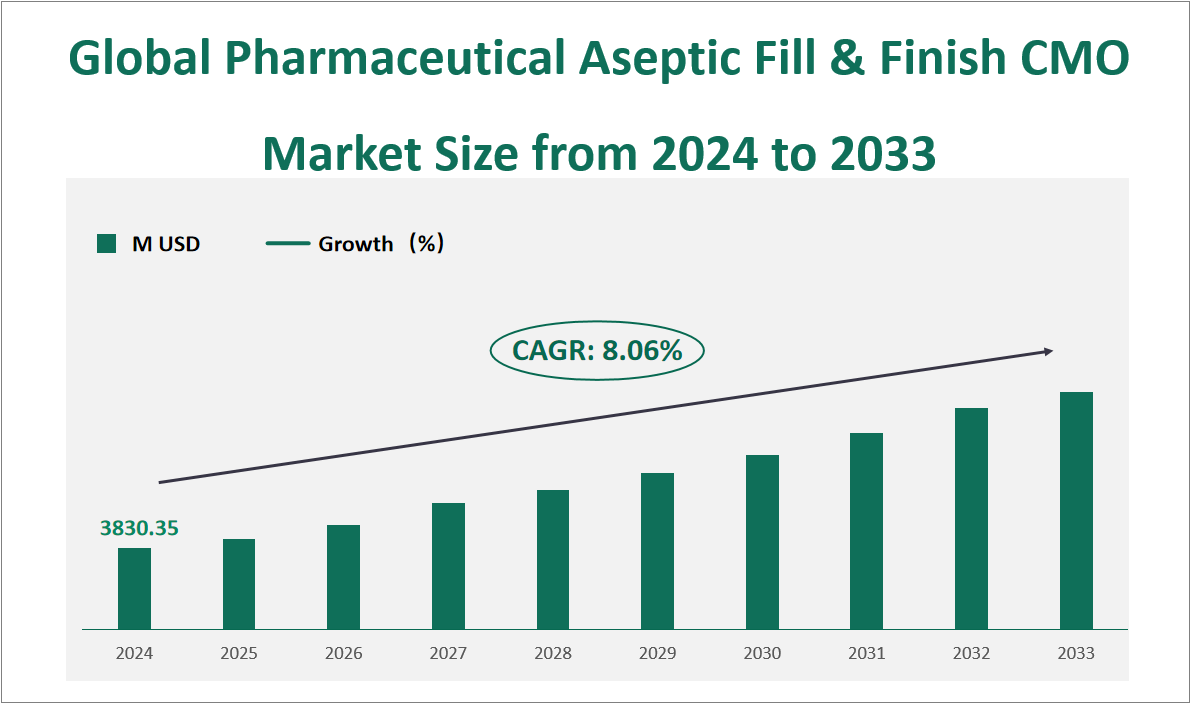
2 Pharmaceutical Aseptic Fill & Finish CMO Market Growth Drivers and Constraints
The growth of the Pharmaceutical Aseptic Fill & Finish CMO market is driven by several key factors. Firstly, advancements in aseptic equipment and processes have significantly improved the efficiency and safety of production. Modern aseptic fill/finish systems are designed to minimize contamination risks, ensuring that pharmaceutical products meet stringent regulatory standards. This technological progress has not only enhanced product quality but also reduced production costs, making these services more accessible to a broader range of pharmaceutical companies.
Another significant driver is the increasing number of biologic drugs in the market. As the demand for biologics grows, so does the need for specialized aseptic fill/finish services. These drugs, which include vaccines, monoclonal antibodies, and cell and gene therapies, require highly controlled manufacturing environments to maintain their efficacy and safety. The rise in the number of biologics has thus spurred the demand for contract manufacturing services, particularly in the aseptic fill/finish segment.
The COVID-19 pandemic has also played a crucial role in driving market growth. The rapid development and deployment of vaccines have highlighted the importance of aseptic fill/finish capabilities. The need for large-scale production of vaccines has led to increased investment in this area, further boosting the market.
However, the market also faces several limiting factors. One of the most significant challenges is the strict regulatory environment. Aseptic fill/finish processes are subject to rigorous oversight by regulatory bodies such as the FDA and EMA. Compliance with these standards requires substantial investment in quality control and validation, which can be a barrier for some companies.
Another challenge is the shortage of skilled technicians. The aseptic fill/finish process requires highly trained professionals who can operate complex equipment and maintain sterile conditions. The limited availability of such skilled workers can hinder the expansion of CMOs and limit market growth.
3 Pharmaceutical Aseptic Fill & Finish CMO Market Innovations and M&A Activities
Technological innovation has been a cornerstone of the Pharmaceutical Aseptic Fill & Finish CMO market. Companies are continuously investing in advanced technologies to improve the efficiency, safety, and scalability of their operations. Automation and robotics are increasingly being integrated into fill/finish lines, reducing human error and enhancing productivity. The adoption of single-use technologies and advanced sterilization methods has also contributed to the improvement of aseptic conditions and overall product quality.
Corporate mergers and acquisitions have also played a significant role in shaping the market. Major players such as Baxter BioPharma Solutions, Vetter Pharma, and Aenova have expanded their capabilities through strategic acquisitions. For example, Vetter Pharma acquired a clinical manufacturing site in Austria to enhance its fill/finish capacity. Similarly, Ajinomoto Bio-Pharma Services completed the acquisition of Granules OmniChem Joint Venture in India, strengthening its global presence.
These strategic moves have not only expanded the capabilities of individual companies but also increased competition within the market. As companies continue to invest in R&D and seek new opportunities for growth, the market is expected to become more dynamic and innovative.
In conclusion, the global Pharmaceutical Aseptic Fill & Finish CMO market is poised for continued growth, driven by technological advancements, increasing demand for biologic drugs, and the impact of the COVID-19 pandemic. While regulatory challenges and a shortage of skilled workers pose significant hurdles, companies are actively addressing these issues through innovation and strategic partnerships. As the market evolves, it will be essential for companies to stay ahead of technological trends and regulatory requirements to maintain a competitive edge and capitalize on emerging opportunities.
4 Global Pharmaceutical Aseptic Fill & Finish CMO Market Analysis by Type
In 2024, the global Pharmaceutical Aseptic Fill & Finish CMO market is projected to have a total revenue of $3830.85 million. By type, the market is segmented into Ampoule Filling Services, Vial Filling Services, Aseptic Prefilled Syringes/Sterile Syringe Filling Services, and Others. Specifically, Ampoule Filling Services are expected to generate a revenue of $1186.30 million, accounting for 30.97% of the total market revenue. Vial Filling Services are anticipated to contribute $2016.07 million, holding a market share of 52.63%. Aseptic Prefilled Syringes/Sterile Syringe Filling Services are forecasted to produce $393.48 million in revenue, representing 10.27% of the total. Lastly, the segment classified as Others is expected to yield $234.99 million, with a market share of 6.13%. These figures highlight the significant contributions of Vial Filling Services and Ampoule Filling Services to the overall market revenue, while also indicating the growing importance of Aseptic Prefilled Syringes/Sterile Syringe Filling Services in the market.
Table Global Pharmaceutical Aseptic Fill & Finish CMO Market Size and Share by Type in 2024
Type | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Ampoule Filling Services | 1186.30 | 30.97% |
Vial Filling Services | 2016.07 | 52.63% |
Aseptic Prefilled Syringes/Sterile Syringe Filling Services | 393.48 | 10.27% |
Others | 234.99 | 6.13% |
5 Global Pharmaceutical Aseptic Fill & Finish CMO Market Analysis by Application
In 2024, the global Pharmaceutical Aseptic Fill & Finish CMO market is projected to have a total consumption value of $3830.85 million. The market is segmented by application into Vaccines, Commercial Drug products, Clinical Drug products, Antibiotics, Diagnostics and Reagents, and Others. Specifically, the consumption value for Vaccines is expected to be $725.00 million, representing 18.93% of the total market. Commercial Drug products are anticipated to contribute $638.76 million, holding a market share of 16.67%. Clinical Drug products are forecasted to have a consumption value of $1489.20 million, accounting for 38.87% of the total market. Antibiotics are expected to generate $304.59 million, representing 7.95% of the total. Diagnostics and Reagents are projected to contribute $449.58 million, with a market share of 11.74%. Lastly, the segment classified as Others is expected to have a consumption value of $223.73 million, holding a market share of 5.84%. These figures highlight the significant contributions of Clinical Drug products and Vaccines to the overall market consumption value, while also indicating the diverse range of applications within the Pharmaceutical Aseptic Fill & Finish CMO market.
Table Global Pharmaceutical Aseptic Fill & Finish CMO Market Size and Share by Application in 2024
Application | Market Size in 2024 (M USD) | Market Share in 2024 (%) |
|---|---|---|
Vaccines | 725.00 | 18.93% |
Commercial Drug products | 638.76 | 16.67% |
Clinical Drug products | 1489.20 | 38.87% |
Antibiotics | 304.59 | 7.95% |
Diagnostics and Reagents | 449.58 | 11.74% |
Others | 223.73 | 5.84% |
6 Global Pharmaceutical Aseptic Fill & Finish CMO Market Analysis by Region
In 2024, the global Pharmaceutical Aseptic Fill & Finish CMO market is projected to generate a total revenue of $3830.85 million. Regionally, the United States is expected to lead with a revenue of $1251.84 million, holding a market share of 32.68%. Europe follows closely with a revenue of $1169.71 million, accounting for 30.54% of the total market. China is anticipated to contribute significantly with a revenue of $541.68 million, representing 14.14% of the market. Japan is projected to have a revenue of $199.13 million, holding a market share of 5.20%. India is expected to generate $157.65 million, contributing 4.12% to the total revenue. Southeast Asia is forecasted to have a revenue of $21.97 million, with a market share of 0.57%. Latin America is expected to contribute $209.14 million, holding a market share of 5.46%. The Middle East and Africa are projected to generate $130.63 million, accounting for 3.41% of the market. These regional dynamics highlight the significant contributions of the United States and Europe, while also indicating the growing importance of emerging markets like China and India in the global Pharmaceutical Aseptic Fill & Finish CMO industry.
Figure Global Pharmaceutical Aseptic Fill & Finish CMO Market Share by Region in 2024
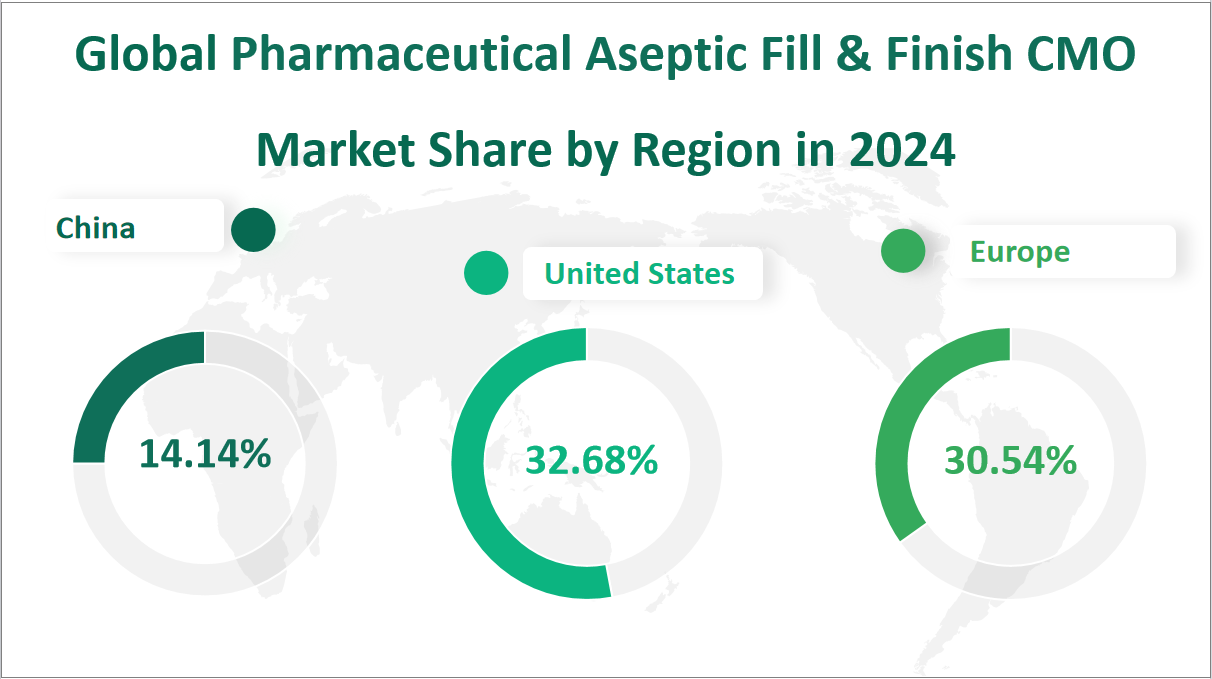
7 Top 3 Companies of Global Pharmaceutical Aseptic Fill & Finish CMO Market
7.1 Baxter BioPharma Solutions
Company Introduction and Business Overview:
Baxter BioPharma Solutions is a leading provider of aseptic fill and finish services, specializing in the production of sterile injectable pharmaceuticals. Established in 1931, Baxter has a long history of innovation and excellence in the healthcare industry. The company operates globally, with a strong presence in North America, Europe, and Asia-Pacific. Baxter BioPharma Solutions is known for its commitment to quality and compliance with stringent regulatory standards, ensuring that its products meet the highest levels of safety and efficacy.
Products Offered:
Baxter BioPharma Solutions offers a comprehensive range of aseptic fill and finish services, including the production of prefilled syringes, vials, and ampoules. The company’s state-of-the-art facilities are equipped with advanced technologies to ensure the highest quality and sterility of pharmaceutical products. Baxter’s services cover the entire spectrum of aseptic manufacturing, from clinical trial supplies to commercial-scale production. The company also provides specialized services such as lyophilization (freeze-drying) and terminal sterilization, which are critical for maintaining the stability and shelf life of pharmaceutical products.
Sales Revenue in the Latest Year:
Baxter BioPharma Solutions achieved a revenue of $672.04 million. This growth reflects the company’s strong market position and its ability to meet the increasing demand for high-quality aseptic fill and finish services. Baxter’s continued investment in R&D and facility expansion has enabled it to stay ahead of the competition and deliver innovative solutions to its clients.
7.2 Vetter Pharma
Company Introduction and Business Overview:
Vetter Pharma is a globally recognized Contract Development and Manufacturing Organization (CDMO) specializing in aseptic production of prefilled syringe systems, cartridges, and vials. Founded in 1950, Vetter has built a reputation for excellence in the pharmaceutical industry, particularly in the area of aseptic fill and finish. The company operates multiple state-of-the-art facilities in Germany and the United States, offering a wide range of services from early-stage development to commercial manufacturing. Vetter’s commitment to innovation and quality has made it a preferred partner for many leading pharmaceutical and biotech companies.
Products Offered:
Vetter Pharma provides a comprehensive suite of aseptic fill and finish services, including the production of prefilled syringes, vials, and cartridges. The company’s advanced facilities are equipped with the latest technologies to ensure the highest levels of sterility and quality control. Vetter’s services cover the entire product lifecycle, from clinical trial supplies to large-scale commercial production. The company also offers specialized services such as lyophilization and terminal sterilization, which are essential for maintaining the stability and efficacy of pharmaceutical products.
Sales Revenue in the Latest Year:
Vetter Pharma reported a revenue of $549.46 million. This growth is attributed to the company’s strong market position, innovative technologies, and strategic expansions. Vetter’s continued investment in R&D and facility upgrades has enabled it to meet the evolving needs of its clients and maintain a competitive edge in the market.
7.3 Aenova
Company Introduction and Business Overview:
Aenova is a leading provider of aseptic fill and finish services, specializing in the production of sterile pharmaceutical products. Established in 2008, Aenova has quickly become a prominent player in the pharmaceutical industry, particularly in the areas of aseptic fill and finish. The company operates multiple facilities in Germany and Italy, offering a wide range of services from early-stage development to commercial manufacturing. Aenova’s commitment to quality and innovation has made it a trusted partner for many pharmaceutical companies.
Products Offered:
Aenova offers a comprehensive range of aseptic fill and finish services, including the production of vials, ampoules, and prefilled syringes. The company’s state-of-the-art facilities are equipped with advanced technologies to ensure the highest levels of sterility and quality control. Aenova’s services cover the entire product lifecycle, from clinical trial supplies to large-scale commercial production. The company also provides specialized services such as lyophilization and terminal sterilization, which are essential for maintaining the stability and efficacy of pharmaceutical products.
Sales Revenue in the Latest Year:
Aenova achieved a revenue of $281.79 million. This growth reflects the company’s strong market position and its ability to meet the increasing demand for high-quality aseptic fill and finish services. Aenova’s continued investment in R&D and facility expansion has enabled it to stay ahead of the competition and deliver innovative solutions to its clients.

