1 Global Testing Swab Kits Market Insight Analysis
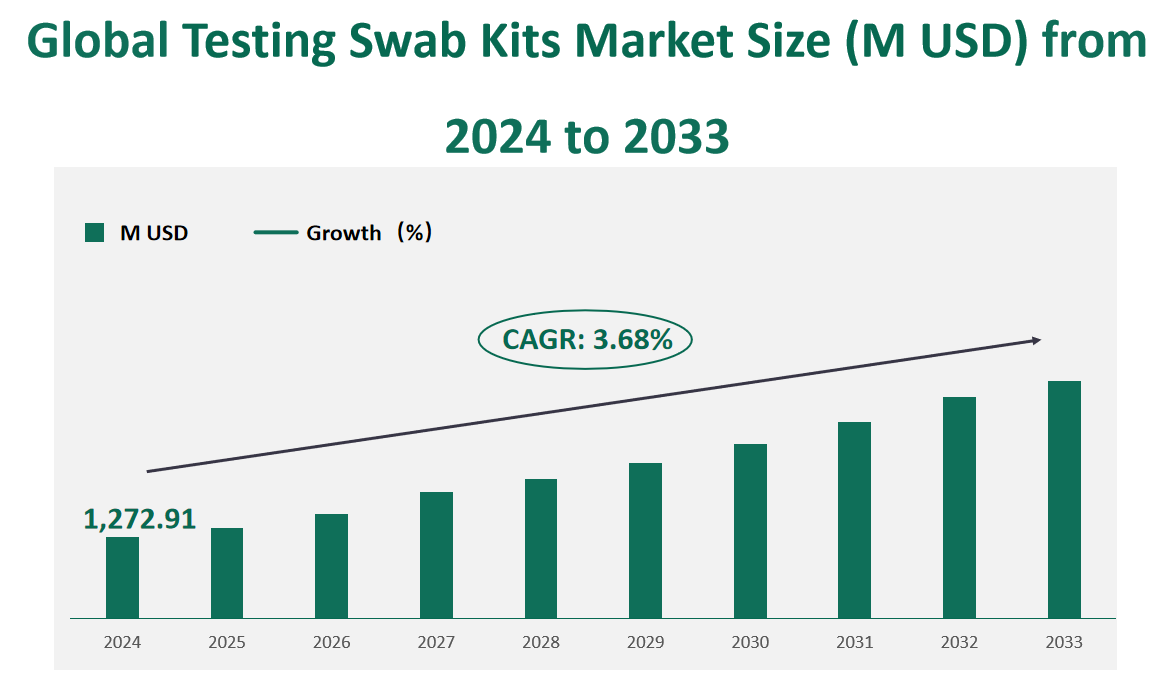
The global Testing Swab Kits market is valued at USD 1,272.91 million in 2024 and is expected to register a CAGR of approximately 3.68% during the period 2024-2033.
Testing swab kits are essential tools used for collecting biological samples for diagnostic purposes. These kits typically include swabs made from materials such as nylon or polyester, designed to effectively collect and preserve samples for transport to laboratories. They are crucial in various medical applications, including viral and bacterial testing, and have played a vital role in the global response to COVID-19.
Figure Global Testing Swab Kits Market Size (M USD) and CAGR (2024-2033)
2 Testing Swab Kits Market Growth Drivers and Restraints
Economic development and the improvement of people’s health care awareness have led to a continuous increase in downstream demand. On the one hand, people pay more attention to their own health and are willing to take the initiative to undergo regular physical examinations, which has led to a continuous increase in the demand for test swab kits in the field of medical testing; on the other hand, economic development has led to a significant increase in social investment in biomedical research, and the demand for test swab kits in various studies has also increased accordingly, which has strongly promoted the growth of the market.
Product innovation is one of the core driving forces for the development of the industry. Early cotton swabs had many disadvantages, such as the inability to effectively release specimens, interference with microbiology and DNA extraction processes, etc. In order to meet market demand, merchants actively developed new products, such as Rayon swabs and flocking swabs. These new products have good sample collection and release performance, greatly improving the safety of samples and the accuracy of testing, thereby promoting the development of the industry.
The rise of the Internet has brought new development opportunities to the test swab kit market. By building their own websites, companies can fully display brand culture and product characteristics, making it convenient for potential customers and distributors to understand product information, inquire about prices, and provide after-sales feedback. This not only enhances the company’s visibility, but also shortens the distance between the company and its customers, thereby promoting product sales and driving market growth.
The industry has a high market concentration, and the growth of downstream demand has attracted many companies to participate in the competition. In order to gain competitive advantages, companies are competing fiercely in pricing, user experience, value-added services, etc. For companies that already have a stable customer base, a mature team and rich market experience, the impact may be relatively small; but new entrants that lack a strong team, sufficient funds and a sound sales channel will face huge market competition pressure and find it difficult to gain a foothold in the market.
The development of the world economy is highly dependent on the stability of world trade, and factors such as geopolitical turmoil, changes in the EU system, and trade wars have led to global economic instability and intensified trade frictions. Taking the Sino-US trade war as an example, the two countries have imposed tariffs on each other, which has seriously affected the interests of companies on both sides, inhibited the development of the global economy, and also had an impact on the international trade of test swab kits, increasing market uncertainty.
With the development of the epidemic, the export barriers of test swab kits have increased significantly. Enterprises not only need to obtain domestic registration certificates, but also overseas registration licenses before they can export products. This reduces the number of enterprises that can participate in export business and limits the space for market expansion.
3 Technological Innovations in the Testing Swab Kits Market
To address the shortcomings of traditional swabs, new swab products are constantly being launched on the market. For example, the 3D printed sterile nasal swab CentoSwab developed by Resolution Medical uses innovative 3D printing technology, approved existing materials and carbon digital photosynthesis technology for rapid production. Its unique design, such as a 5.9-inch length, a soft lattice cage swab head surrounding a soft spiral core, and a unique lattice dome tip, not only improves the comfort and efficiency of sample collection, but also ensures that enough samples are collected for testing, providing the market with better testing tools.
Various companies are committed to improving the performance of test swab kits. For example, Thermo Fisher Scientific’s MicroTest™ series of products have been carefully designed from formulation to packaging, which can maintain the vitality of organisms during freeze-thaw cycles, while inhibiting antimicrobial contaminants and improving the safety of samples; Copan Group’s ESwab™ uses a patented liquid microbial sample collection and transportation system, combined with flocked swabs and 1mL liquid Amies, which has been tested to fully comply with relevant standards, is suitable for the collection and transportation of a variety of bacteria, and can ensure the activity of samples for a certain period of time.
On March 3, 2020, Thermo Fisher Scientific acquired Qiagen, and both parties focused on complementary products for future clinical diagnostics and life science research support businesses. Qiagen has advantages in detecting latent tuberculosis and infectious diseases, while Thermo Fisher has extensive experience in oncology, allergy and autoimmune testing, and transplant diagnostics. This acquisition will help both parties integrate technology and resources, further expand the market, and enhance testing capabilities and product competitiveness in related fields.
On April 8, 2021, Hologic acquired Mobidiag. Mobidiag is able to provide testing services for detecting multiple pathogens from a single test sample, which enables Hologic to enter the acute care testing market, enrich its product line, enhance its comprehensive strength in the field of medical testing, and win a more advantageous position for it in market competition.
4 Global Testing Swab Kits Market Size by Type
Nasopharyngeal swabs are designed for collecting samples from the nasopharynx, a critical area for detecting respiratory infections, including COVID-19. In 2024, the market value for NP swabs is expected to be USD 439.81 million. The demand for NP swabs is driven by their effectiveness in detecting pathogens in the upper respiratory tract, making them essential in both pandemic and non-pandemic settings.
Oropharyngeal swabs are used for collecting samples from the oropharynx, which is crucial for diagnosing infections affecting the throat and oral cavity. In 2024, the market value for OP swabs is forecasted to be USD 583.14 million. Their versatility and wide application in various diagnostic tests contribute to their significant market share.
Nasal swabs are designed for collecting samples from the nasal cavity. They are simpler to use and less invasive compared to NP swabs, making them suitable for routine screening and testing. In 2024, the market value for nasal swabs is projected to be USD 140.94 million. The demand for nasal swabs is driven by their ease of use and effectiveness in detecting pathogens in the nasal cavity.
Table Global Testing Swab Kits Market Size by Type in 2024
Type | Market Size (M USD) 2024 |
|---|---|
Nasopharyngeal (NP) Swab | 439.81 |
Oropharyngeal (OP) Swab | 583.14 |
Nasal Swab | 140.94 |
Others | 109.01 |
5 Global Testing Swab Kits Market Size by Application
In 2024, the global testing swab kits market value by application is expected to show different trends. Laboratories are projected to have a market value of $197.96 million. The growth of the laboratory segment is driven by increasing research activities in the biomedical field. With more scientific research projects focused on disease diagnosis and treatment, the demand for accurate sample collection using testing swab kits in laboratories is on the rise.
Hospitals are expected to have a market value of $669.04 million. The high demand in hospitals is due to the continuous need for patient diagnosis and monitoring. As the healthcare industry expands and the number of patients seeking medical services grows, hospitals require a large number of testing swab kits for routine and emergency tests.
Diagnostic centers and clinics are forecasted to reach a market value of $336.96 million. These facilities play a crucial role in providing quick and accurate diagnostic services, and thus rely heavily on testing swab kits.
Table Global Testing Swab Kits Market Size by Application in 2024
Application | Market Size (M USD) 2024 |
|---|---|
Laboratories | 197.96 |
Hospitals | 669.04 |
Diagnostic Centers and Clinics | 336.96 |
Others | 68.95 |
6 Global Testing Swab Kits Market Size by Region
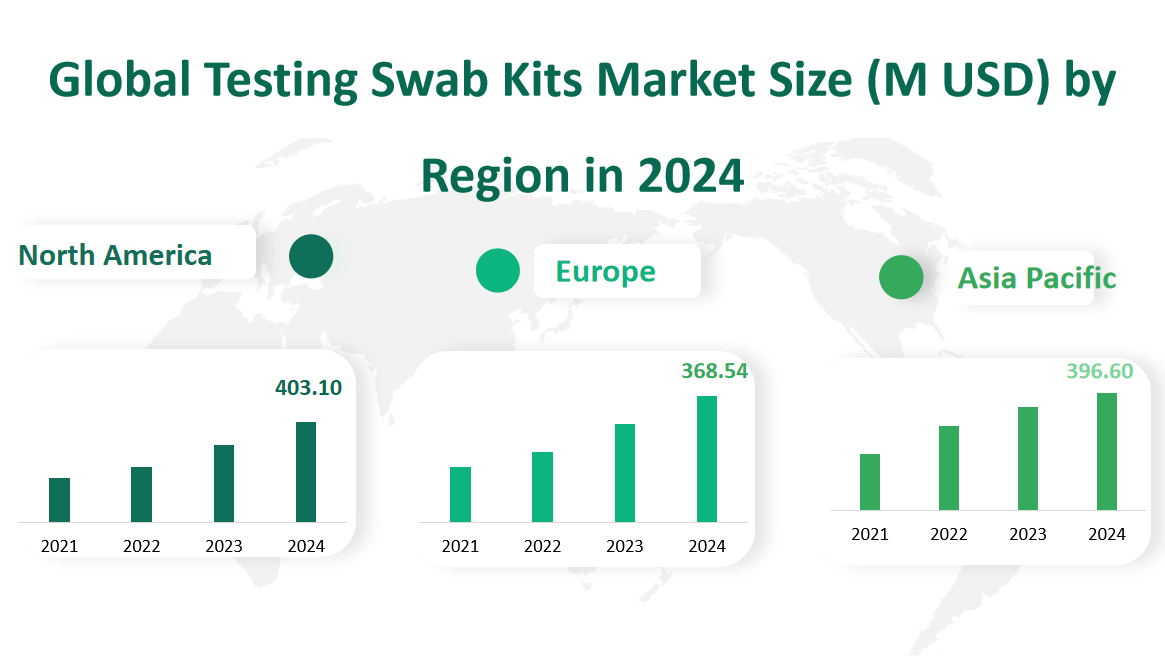
In 2024, the North American testing swab kits market is expected to reach a value of $403.10 million. The region has a well – developed healthcare and research infrastructure. The United States, a major contributor in North America, has a large number of hospitals, laboratories, and research institutions. The high demand for testing swab kits in North America is also driven by the region’s advanced medical technology and the large – scale implementation of disease prevention and control measures. However, the market growth may be affected by factors such as high labor costs and intense competition among market players.
The European market is projected to be valued at $368.54 million in 2024. Europe has a long – established healthcare system with a strong emphasis on medical research and development. Countries like Germany, the UK, and France have a high demand for testing swab kits in hospitals and diagnostic centers. The region’s market is also influenced by strict regulatory requirements for medical devices, which ensure product quality but may also pose challenges for new entrants. Moreover, the economic recovery in some European countries after the COVID – 19 pandemic is expected to support the growth of the testing swab kits market.
The Asia – Pacific market is expected to reach $396.60 million in 2024. This region has a large population, which provides a huge potential market. China, in particular, has a significant influence on the Asia – Pacific market. With the continuous improvement of China’s healthcare system and the increasing investment in medical research, the demand for testing swab kits has been growing rapidly. Additionally, emerging economies in Southeast Asia are also experiencing growth in the healthcare sector, further driving the market expansion. However, the market in Asia – Pacific also faces challenges such as differences in regulatory environments among countries and competition from local and international manufacturers.
The Middle East and Africa market is forecasted to be worth $39.96 million in 2024. The market in this region has been growing steadily, mainly driven by the increasing awareness of healthcare and the government’s investment in improving the healthcare infrastructure. Countries like Saudi Arabia, UAE, and Egypt are actively promoting healthcare development, which has led to an increased demand for testing swab kits. However, the market may be affected by factors such as geopolitical instability and economic fluctuations in some areas.
The South American market is expected to reach $64.71 million in 2024. Brazil is the largest market in South America, with a significant demand for testing swab kits due to its large population and the development of the healthcare industry. The region’s market growth is also influenced by the increasing investment in public health and the growing awareness of disease prevention. However, challenges such as economic instability and the impact of the COVID – 19 pandemic on the local manufacturing industry may limit the market’s growth rate.
Figure Global Testing Swab Kits Market Size (M USD) by Region in 2024
7 Global Testing Swab Kits Market Analysis by Major Players
7.1 Thermo Fisher Scientific
Company Introduction and Business Overview:
Thermo Fisher Scientific, established in 2006 and headquartered in Waltham, Massachusetts, is a leading supplier of scientific instruments, reagents, and consumables for healthcare, life sciences, and other laboratory applications. The company operates globally, with a strong presence in the United States and extensive distribution networks worldwide.
Thermo Fisher Scientific provides a comprehensive range of products and services for healthcare, life sciences, and industrial applications. Their offerings include analytical instruments, laboratory equipment, and consumables designed to support research, development, and diagnostic activities. The company is known for its innovative solutions and commitment to advancing scientific discovery and healthcare outcomes.
Products:
Thermo Fisher Scientific offers a variety of testing swab kits, including the MicroTest™ Flocked Swab Kits. These kits are specifically designed for the collection, transportation, and long-term storage of virus specimens, chlamydia, mycoplasma, and ureaplasma. The kits maintain the viability of organisms through freeze-thaw cycles and inhibit antibacterial pollutants, ensuring sample integrity and safety.
Market Performance in 2021:
In 2021, Thermo Fisher Scientific reported a revenue of USD 175.87 million from the testing swab kits segment, with a gross margin of 34.31%.
7.2 BD
Company Introduction and Business Overview:
BD, founded in 1897 and headquartered in Franklin Lakes, New Jersey, is a leading global medical technology company. BD specializes in manufacturing and selling medical equipment, instrument systems, and reagents for healthcare providers, researchers, and industrial users.
BD is renowned for its innovative medical technologies and solutions that improve healthcare delivery and patient outcomes. The company operates in various segments, including medical devices, diagnostics, and life sciences. BD’s products are used in hospitals, clinics, research institutions, and industrial settings worldwide.
Products:
BD offers the ESwab™ collection and transportation system, which is designed to collect clinical specimens containing aerobic, anaerobic, and difficult-to-cultivate bacteria. The system ensures the safe transport of samples to laboratories for bacterial culture and analysis, making it a critical tool in diagnostic microbiology.
Market Performance in 2021:
In 2021, BD achieved a revenue of USD 157.08 million from the testing swab kits segment, with a gross margin of 36.48%.
7.3 Orasure Technologies
Company Introduction and Business Overview:
Orasure Technologies, established in 1987 and headquartered in Bethlehem, Pennsylvania, is a leading company in the medical device industry. The company is known for its innovative diagnostic test kits and products designed to improve healthcare outcomes.
Orasure Technologies focuses on developing and manufacturing diagnostic products for various healthcare applications. The company’s products are designed to meet the needs of healthcare providers, researchers, and patients. Orasure Technologies is committed to innovation and improving the accessibility and accuracy of diagnostic testing.
Products:
Orasure Technologies offers the ORAcollect®•RNA product, which is designed for the quick and reliable collection of RNA from saliva samples. This product stabilizes human, viral, and bacterial RNA at room temperature and is compatible with downstream applications such as RT-qPCR and microarray analysis.
Market Performance in 2022:
In 2021, Orasure Technologies reported a revenue of USD 123.73 million from the testing swab kits segment, with a gross margin of 38.83%.

