1 Global Balloon Aortic Valvuloplasty Devices Market Size (Value) and CAGR (2024-2033)
In 2024, the global Balloon Aortic Valvuloplasty Devices market was valued at USD 490.08 million, with a CAGR of 15.4% from 2024 to 2033.
A balloon aortic valvuloplasty (BAV) is a procedure which stretches the aortic valve to improve the symptoms of aortic stenosis. A catheter (thin, flexible tube) is inserted into an artery in groin, and a special dye is then used so that aortic valve will show up on X-rays. A small balloon is then inflated in the aortic valve, which helps to increase blood flow through the heart. This report studies the global balloon aortic valvuloplasty devices.
Figure Global Balloon Aortic Valvuloplasty Devices Market Size (M USD) and CAGR 2024-2033

2 Balloon Aortic Valvuloplasty Devices Market Drivers
The global market for Balloon Aortic Valvuloplasty Devices is experiencing robust growth, driven by several key factors that are reshaping the healthcare landscape. One of the most significant drivers is the increasing demand from an aging population. As the global population continues to age, the prevalence of aortic stenosis—a condition where the aortic valve narrows and restricts blood flow—has risen dramatically. By 2050, one-sixth of the world’s population is projected to be over 65 years old, with the number of individuals aged 80 and above expected to triple. This demographic shift has led to a surge in demand for effective treatments, making Balloon Aortic Valvuloplasty Devices increasingly relevant in addressing cardiovascular health issues.
Another critical driver is the advancement in transcatheter technologies. The advent of transcatheter aortic valve implantation (TAVI) has revolutionized the treatment of aortic stenosis. Modern balloon valvuloplasty devices now benefit from innovations such as improved guidewire technology, enhanced imaging capabilities through CT scans, and more anatomically precise balloon designs. These advancements not only improve procedural outcomes but also reduce the risk of complications, making balloon aortic valvuloplasty a safer and more effective option for patients.
Government policies and regulatory frameworks also play a crucial role in driving market growth. In regions with stable political environments, supportive policies have encouraged the development and adoption of medical devices. For instance, the United States has recently repealed a 2.3% medical device tax, which had previously hindered industry growth. Similarly, China has implemented a unique identification system for medical devices to enhance regulatory oversight and promote innovation. These policy changes create a favorable environment for the expansion of the Balloon Aortic Valvuloplasty Devices market.
3 Balloon Aortic Valvuloplasty Devices Market Restraints
Despite the promising growth prospects, the Balloon Aortic Valvuloplasty Devices market faces several challenges and development constraints that could hinder its expansion. One of the primary concerns is the risk of complications post-procedure. Patients undergoing balloon aortic valvuloplasty may experience a range of adverse events, including vascular hemorrhage, bradycardia, tamponade, aortic annular rupture, and acute heart failure. These complications not only pose significant health risks but also increase the overall cost of treatment and may lead patients to seek alternative therapies. The potential for severe complications necessitates careful patient selection and stringent procedural protocols, which can limit the widespread adoption of the procedure.
Another significant challenge is the intense competition within the industry. The market for Balloon Aortic Valvuloplasty Devices is highly concentrated, with a few major players dominating the landscape. Companies such as Edwards Lifesciences, NuMED, and BD have established strong market positions through continuous innovation and strategic marketing. New entrants face significant barriers to entry, including high research and development costs, stringent regulatory requirements, and the need to differentiate their products in a crowded market. This intense competition can lead to aggressive pricing strategies, reduced profit margins, and increased pressure to innovate constantly.
4 Global Balloon Aortic Valvuloplasty Devices Market Size and Share by Type in 2024
The Balloon Diameter (mm) 18-22 segment is projected to reach a market value of $88.83 million in 2024. These devices are characterized by their smaller diameter, making them particularly suitable for pediatric and young adult patients. The aortic valves in these younger populations are generally smaller, necessitating precision and delicacy in treatment. Devices in this category are often used in minimally invasive procedures, leveraging advanced imaging technologies to ensure precise placement and optimal outcomes. Their smaller size allows for effective dilation without the risk of over-expansion, which is crucial in younger patients where long-term valve function is a priority.
The Balloon Diameter (mm) 22-25 segment is expected to achieve a market value of $147.29 million in 2024. These mid-range devices offer a balance between precision and effectiveness, catering to a broader patient demographic. They are commonly used for middle-aged patients and those with moderate aortic valve stenosis. The versatility of these devices makes them suitable for a wide range of clinical applications, including both standalone procedures and as part of a larger treatment plan. Their mid-range size allows for effective dilation while minimizing the risk of complications, making them a popular choice among healthcare providers.
The largest segment by value is the Balloon Diameter (mm) 25-28, projected to reach $210.44 million in 2024. These larger-diameter devices are specifically designed for older adults and patients with severe aortic stenosis. The larger balloon size is necessary to achieve effective dilation in cases where the aortic valve has become significantly narrowed due to calcification or other age-related factors. These devices are often used in transcatheter aortic valve replacement (TAVR) procedures, both as a standalone treatment and as a pre-dilatation step. Their robust design and larger surface area make them ideal for addressing more complex and severe cases of aortic valve stenosis, where traditional surgical interventions may not be feasible.
Table Global Balloon Aortic Valvuloplasty Devices Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
Balloon Diameter (mm) 18-22 | 88.83 | 18.13% |
Balloon Diameter (mm) 22-25 | 147.29 | 30.05% |
Balloon Diameter (mm) 25-28 | 210.44 | 42.94% |
Others | 43.52 | 8.88% |
5 Global Balloon Aortic Valvuloplasty Devices Market Size and Share by Application in 2024
In 2024, the market value for old man is projected to reach $430.31 million. This segment represents the largest share of the market, driven by the increasing prevalence of aortic stenosis in the aging population. Elderly patients often suffer from severe aortic stenosis, necessitating the use of larger-diameter balloons (25-28 mm) to achieve effective dilation. These devices are crucial in transcatheter aortic valve replacement (TAVR) procedures, where minimally invasive interventions are preferred to reduce surgical risks and recovery times.
The middle-aged patients segment is expected to achieve a market value of $41.76 million in 2024. Middle-aged individuals often present with moderate aortic stenosis, requiring mid-range balloon diameters (22-25 mm) for treatment. This segment benefits from the versatility of balloon aortic valvuloplasty devices, which can be used both as standalone treatments and in combination with other procedures. The demand in this segment is driven by the need for effective interventions that balance precision and safety.
The youth and children segment is projected to reach a market value of $18.02 million in 2024. This segment focuses on pediatric patients and young adults with congenital heart defects or early-onset aortic stenosis. Smaller-diameter balloons (18-22 mm) are essential for treating these patients, as they require precise and delicate interventions to avoid long-term complications. The growth in this segment is driven by advancements in pediatric cardiology and the increasing availability of minimally invasive treatments for younger patients.
Table Global Balloon Aortic Valvuloplasty Devices Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
Old Man | 430.31 | 87.80% |
Middle-aged Person | 41.76 | 8.52% |
Youth and Children | 18.02 | 3.68% |
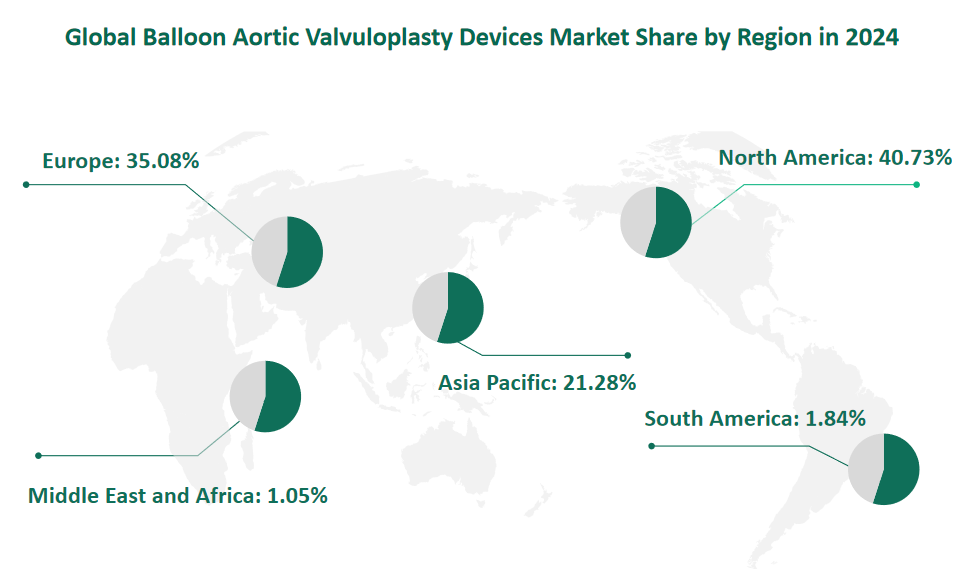
6 Global Balloon Aortic Valvuloplasty Devices Market Size by Region in 2024
North America is projected to hold the largest market value, reaching $199.62 million in 2024. This region is characterized by advanced healthcare infrastructure, high adoption rates of minimally invasive procedures, and a significant aging population. The United States, in particular, is a major driver of the market, supported by robust medical device regulations and a focus on innovation. The demand for high-quality, effective treatments for aortic stenosis is particularly strong, driving the growth of the balloon aortic valvuloplasty devices market.
Europe is expected to achieve a market value of $171.94 million in 2024. This region benefits from a well-developed healthcare system and a strong emphasis on patient care. The market is driven by the increasing prevalence of cardiovascular diseases among the aging population, particularly in Western Europe. Countries such as Germany, France, and the United Kingdom are significant contributors to the market, with a high demand for advanced medical technologies and minimally invasive procedures.
The Asia-Pacific region is projected to reach a market value of $104.31 million in 2024. This region is characterized by rapid economic growth, increasing healthcare spending, and a growing aging population. China and Japan are the major drivers of the market in this region, with significant investments in medical technology and healthcare infrastructure. The demand for balloon aortic valvuloplasty devices is expected to grow rapidly as the region continues to adopt advanced medical technologies and improve healthcare access.
Figure Global Balloon Aortic Valvuloplasty Devices Market Share by Region in 2024
7 Major Players in Global Balloon Aortic Valvuloplasty Devices Market
7.1 Edwards Lifesciences
Company Profile: Edwards Lifesciences is a global leader in patient-focused medical innovations for structural heart disease and critical care monitoring. Established in 1958 and headquartered in the United States, the company is renowned for its commitment to improving and enhancing lives through partnerships with clinicians and stakeholders across the global healthcare landscape. Edwards Lifesciences’ product portfolio includes a wide range of transcatheter heart valve solutions, including the Ascendra Balloon Aortic Valvuloplasty Catheter, which is specifically designed for valvuloplasty procedures.
Business Overview: Edwards Lifesciences is driven by a passion for patients and a dedication to improving cardiovascular health. The company’s business strategy focuses on continuous innovation, strategic partnerships, and a deep understanding of clinical needs. With a global presence and a strong commitment to research and development, Edwards Lifesciences aims to provide effective and minimally invasive solutions for structural heart disease.
Product and Service Analysis: The Ascendra Balloon Aortic Valvuloplasty Catheter is one of Edwards Lifesciences’ flagship products. It is indicated for valvuloplasty of stenotic aortic valves prior to the implantation of transcatheter heart valves. The device features high precision and reliability, making it suitable for both standalone procedures and as part of a larger treatment plan. Edwards Lifesciences’ products are known for their quality and innovation, supported by rigorous clinical testing and regulatory approvals.
Recent Financial Performance: In the most recent year, Edwards Lifesciences reported a sales value of $224.83 million from its balloon aortic valvuloplasty devices.
7.2 NuMED
Company Profile: NuMED, founded in 1984 and headquartered in the United States, is a leading developer and manufacturer of minimally invasive cardiovascular products. The company is known for its innovative approach to treating congenital heart defects and has a global presence, distributing its products in over 100 countries. NuMED collaborates with leading medical professionals to develop high-quality devices that improve patient outcomes and long-term health.
Business Overview: NuMED’s business strategy focuses on innovation and collaboration. The company works closely with interventional cardiologists and other healthcare providers to develop products that address unmet clinical needs. NuMED’s commitment to quality and innovation has positioned it as a key player in the global market for cardiovascular devices.
Product and Service Analysis: NuMED’s Z-Med II-X™ catheter is a standout product in the balloon aortic valvuloplasty market. The device is designed for percutaneous transluminal valvuloplasty (PTV) and pre-dilatation in transcatheter heart valve replacement procedures. It features a wide range of sizes (2-40 mm), high burst pressures, and rapid inflation and deflation times, making it suitable for various clinical scenarios. NuMED’s products are known for their versatility and effectiveness, supported by extensive clinical use and positive feedback from healthcare providers.
Recent Financial Performance: In the most recent year, NuMED reported a sales value of $37.68 million from its balloon aortic valvuloplasty devices.
7.3 BD
Company Profile: BD, established in 1897 and headquartered in the United States, is one of the largest global medical technology companies. BD is committed to advancing healthcare by improving medical discovery, diagnostics, and the delivery of care. With a global presence and a wide range of products, BD is a key player in the medical device industry, focusing on innovation and efficiency.
Business Overview: BD’s business strategy centers on improving patient outcomes and enhancing the efficiency of healthcare delivery. The company collaborates with healthcare providers, researchers, and other stakeholders to develop innovative solutions that address critical healthcare challenges. BD’s commitment to quality and innovation has positioned it as a leader in the global medical technology market.
Product and Service Analysis: BD’s True™ Dilatation Balloon Valvuloplasty Catheters are designed for precision and reliability in aortic valvuloplasty procedures. The devices feature rapid inflation and deflation times, high durability, and precise diameter control, making them suitable for a wide range of clinical applications. BD’s products are known for their quality and innovation, supported by extensive clinical testing and regulatory approvals.
Recent Financial Performance: In the most recent year, BD reported a sales value of $31.80 million from its balloon aortic valvuloplasty devices.

