1 Global Pain Monitoring Device Market Insight Analysis
The global pain monitoring device market size is expected to be USD 22.38 million in 2024, with a CAGR of 20.61% from 2024 to 2033.
Pain monitoring devices are devices that quantify a patient’s physiological response to pain. Objectively assessing a person’s level of pain has long been a challenge. The problem is compounded when patients cannot describe their pain. Pain monitoring devices could help doctors objectively assess a patient’s pain in intensive care situations where patients cannot communicate. This enables doctors to ensure pain is being managed properly.
Figure Global Pain Monitoring Device Market Size (M USD) and CAGR (2024-2033)

2 Pain Monitoring Device Market Growth Drivers and Restraints
Increased number of surgeries: The high incidence of chronic diseases (such as cancer and neurological diseases) worldwide has led to a continuous increase in the demand for surgery. The increase in the number of surgeries has directly driven the demand for pain monitoring equipment. During surgery, especially when under general anesthesia, patients cannot subjectively express their pain feelings, while pain monitoring equipment can objectively monitor the patient’s pain response, help doctors evaluate the analgesic effect, optimize the pain management plan, and avoid excessive medication, which is crucial to improving the quality of surgery and the patient’s rehabilitation effect.
The importance of pain monitoring is highlighted: Pain, as a common clinical symptom, is the fifth vital sign of the human body. It not only affects the patient’s physical condition, but may also cause serious risks such as cardiovascular and cerebrovascular accidents. Effective pain monitoring is of great significance to the treatment and rehabilitation of patients. Whether it is acute pain (such as postoperative pain, labor pain) or chronic pain, accurate monitoring methods are required, which provides a broad space for the development of the pain monitoring equipment market.
Lack of accurate and objective standards and not widely used: At present, pain monitoring equipment faces many difficulties in evaluating sedation or analgesia. The working principles and technical means of different devices are different, and there is a lack of a large amount of clinical data to verify their effectiveness and reliability. At the same time, it is difficult to determine the optimal threshold for harmful stimulation, pain assessment is subjective, and there is a lack of objective “gold standard”. This makes it difficult to fully promote and apply pain monitoring equipment under the concept of “precision anesthesia”, and the acceptance of various hospitals varies, which limits the further expansion of the market.
Inflation brings cost pressure: Inflation has caused the prices of raw materials such as metals, plastics, and chemicals to rise, and key components such as semiconductors to be in short supply, which has greatly increased the production costs of the medical equipment industry. Rising transportation costs and longer delivery times also bring additional burdens to companies. Although companies have taken measures such as re-evaluating suppliers and pre-purchasing key materials, they still face challenges in cost control and profit protection.
3 Technological Innovations in the Pain Monitoring Device Market
Various technologies explore new ways of pain monitoring: In order to achieve more accurate pain monitoring, a variety of innovative technologies have emerged in the market. SPI (surgical stress index) assesses pain by monitoring the patient’s hemodynamic response to surgical stimulation and analgesic treatment, but it is dependent on the concentration of remifentanil. Changes in pupil diameter can be used as a pain monitoring indicator.
In children with sevoflurane anesthesia, its sensitivity to harmful stimulation is higher than heart rate and blood pressure, but there are problems such as difficulty in standardizing the measurement environment and lack of judgment threshold in clinical applications. The SC (skin conductance) pain meter uses the analysis of the SC rate to reflect the changes in the sympathetic nervous system caused by pain. It has the advantages of being objective, non-invasive, and responsive. It can be used for pain assessment in neonates, children, and patients with general anesthesia intubation, but research in the neonatal population is still incomplete.
Enterprises actively develop and enhance product competitiveness: Enterprises have invested heavily in technology research and development. Medasense Biometrics’ PMD-200 device uses NOL technology to quantify patients’ physiological pain response and is suitable for operating rooms and intensive care scenarios. Mdoloris Medical Systems’ ANI monitoring technology measures parasympathetic nerve tension to assess patient comfort and assist doctors in titrating analgesics. The iPainfree® pain management information system developed by Jiangsu Apon Medical integrates infusion monitoring, pain assessment, and quality control management. PaMeLa is committed to using AI technology to automatically calculate pain levels based on brain wave patterns and promote the objectivity of pain monitoring.
4 Global Pain Monitoring Device Market Size by Type
In 2024, the market value of pain monitoring devices used in the Operating Room is projected to be 12.16 million US dollars. This is the highest revenue – generating segment among the three. The Operating Room is a critical area where precise pain management is essential during surgical procedures. Pain monitoring devices help anesthesiologists and surgical teams to assess a patient’s pain level accurately.
For example, during major surgeries such as cardiac or orthopedic operations, patients are under anesthesia, and their ability to communicate pain is impaired. These devices provide real – time data on the patient’s physiological responses to pain, enabling the medical team to adjust the dosage of analgesics appropriately. This not only ensures the patient’s comfort but also helps in preventing complications related to under – or over – administration of painkillers.
The market value of pain monitoring devices in the Critical Care segment in 2024 is estimated at 8.52 million US dollars. Critical Care units are home to patients with severe and life – threatening conditions, such as those suffering from trauma, severe burns, or post – cardiac arrest. These patients often experience significant pain, and accurate pain monitoring is crucial for their treatment and recovery. Pain monitoring devices in Critical Care help healthcare providers to manage pain effectively, which in turn can impact the patient’s physiological stability, immune function, and overall prognosis. For instance, in patients with multiple fractures, continuous pain monitoring can guide the adjustment of pain medications to prevent stress – induced complications.
Table Global Pain Monitoring Device Market Size and Share by Type in 2024
Type | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
Operating Room | 12.16 | 54.34% |
Critical Care | 8.52 | 38.06% |
Others | 1.70 | 7.60% |
5 Global Pain Monitoring Device Market Size by Application
Hospitals are the dominant end – users in the global pain monitoring device market. In 2024, the market value of pain monitoring devices for hospitals is projected to reach 20.56 million US dollars. This significant revenue is a reflection of the high demand for these devices in hospital settings. Hospitals handle a large number of patients, including those undergoing surgeries, suffering from chronic pain, or in critical care units. Pain monitoring is essential in these scenarios to ensure proper pain management, which in turn affects patient recovery and overall healthcare quality. For example, during surgical procedures, accurate pain monitoring helps anesthesiologists adjust the dosage of analgesics, preventing both under – and over – medication.
Clinics also play an important role in the market. In 2024, the market value of pain monitoring devices for clinics is estimated to be 1.05 million US dollars. Although this figure is much smaller compared to that of hospitals, it still represents a growing segment. Clinics often deal with out – patient procedures and patients with mild to moderate pain conditions. Pain monitoring devices in clinics assist in providing appropriate pain relief and ensuring patient comfort during treatments such as minor surgeries, physical therapy sessions, or pain management consultations.
Table Global Pain Monitoring Device Market Size and Share by Application in 2024
Application | Market Size (M USD) 2024 | Market Share 2024 |
|---|---|---|
Hospitals | 20.56 | 91.89% |
Clinics | 1.05 | 4.68% |
Others | 0.77 | 3.44% |
6 Global Pain Monitoring Device Market Size by Region
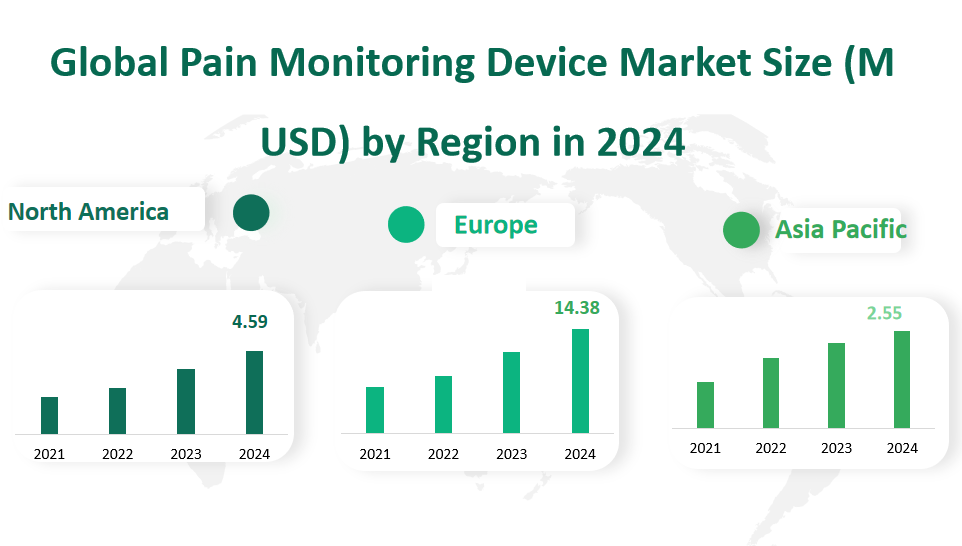
Europe leads in terms of market revenue for pain monitoring devices in 2024, with an estimated value of 14.38 million US dollars. This region has a well – established healthcare infrastructure, with advanced medical facilities and a high prevalence of chronic diseases, which drives the demand for pain management solutions. European countries invest heavily in healthcare research and development, leading to the adoption of advanced pain monitoring technologies. For example, countries like Germany and the United Kingdom have sophisticated healthcare systems that prioritize patient – centric pain management, resulting in a high uptake of pain monitoring devices across hospitals and clinics.
North America follows closely, with a market revenue of 4.59 million US dollars in 2024. The region benefits from a combination of a large aging population, high healthcare spending, and a robust medical technology industry. The United States, in particular, is at the forefront of medical innovation. There is a growing awareness among patients and healthcare providers about the importance of effective pain management, which fuels the demand for pain monitoring devices. Additionally, the presence of major medical device manufacturers and research institutions in North America contributes to the continuous development and adoption of new pain monitoring technologies.
The Asia Pacific region has a market revenue of 2.55 million US dollars in 2024. While it currently lags behind Europe and North America, it is experiencing rapid growth. The increasing population, rising disposable income, and improving healthcare infrastructure across countries like China, India, and Japan are driving the demand for pain monitoring devices. Moreover, governments in the region are investing more in healthcare to meet the growing needs of their populations, which is further boosting the market. For instance, in China, the expansion of hospital networks and the emphasis on quality healthcare services have led to an increased adoption of advanced medical devices, including pain monitoring equipment.
The MEA region has a market revenue of 0.60 million US dollars in 2024. The region is gradually developing its healthcare capabilities, with some countries investing in modernizing their healthcare systems. The demand for pain monitoring devices is rising due to factors such as increasing awareness of pain management and the growth of the private healthcare sector. However, challenges such as limited healthcare budgets in some areas and disparities in access to advanced medical technologies still exist.
Latin America has the lowest market revenue among these regions in 2024, amounting to 0.26 million US dollars. Despite facing challenges such as economic instability in some countries and limited healthcare resources in certain areas, the region shows potential for growth. Improving healthcare access and increasing focus on patient – centered care are driving the adoption of pain monitoring devices, albeit at a slower pace compared to some other regions.
Figure Global Pain Monitoring Device Market Size (M USD) by Region in 2024
7 Global Pain Monitoring Device Market Analysis by Major Players
Medasense Biometrics
Company Profile: Established in 2008, Medasense Biometrics is headquartered in Israel. Its sales regions cover the US, Europe, Canada, South Africa, the UAE, Israel, and parts of Latin America.
Business Overview: Medasense Biometrics is a medical device company that focuses on developing the PMD-200, a continuous and non-invasive pain monitoring device. The initial application of this device is in operating rooms, followed by intensive care units, and eventually in clinics and home care environments to treat chronic pain patients.
Product Offered: The PMD-200™ monitoring device leverages NOL technology to quantify patients’ physiological responses to pain (nociception). Designed for operating rooms and critical care settings, it enables clinicians to assess nociception and objectively titrate analgesics to meet patients’ specific needs with optimized timing and dosage.
2023 Revenue Summary: In 2023, Medasense Biometrics achieved a revenue of 8.47 million USD.
Mdoloris Medical Systems
Company Profile: Founded in 2010, Mdoloris Medical Systems is based in France and has a global sales reach.
Business Overview: The company offers devices that help patients under general anesthesia or unconsciousness communicate their distress to clinicians. These devices assist in titrating analgesics to manage surgical stress and improve outcomes in operating rooms and intensive care units.
Product Offered: The ANI Monitor V2 provides a novel and innovative technology to continuously evaluate patients’ comfort levels. It allows physicians to titrate analgesics to control nociception. The benefits of ANI technology include predicting hemodynamic reactivity, post-extubation pain, refining opioid titration, reducing post-operative pain, aiding in diagnosing the etiology of hemodynamic events, and shortening the length of stay in outpatient surgery units.
2023 Revenue Summary: In 2023, Mdoloris Medical Systems generated a revenue of 6.7 million USD.
Jiangsu Apon Medical
Company Profile: Established in 2001, Jiangsu Apon Medical is headquartered in China, with its sales primarily concentrated in Asia.
Business Overview: Jiangsu Apon Medical is a Chinese company engaged in the R&D, production, and sales of medical devices for pain management and nasal care. Its product portfolio includes microcomputer injection pumps, disposable injection pumps, wireless analgesia management systems, pulse oximeters, sensors, and other medical devices for pain management and nasal care.
Product Offered: The iPainfree® pain management information system/obstetric delivery information management system features infusion monitoring, enabling visualized and centralized monitoring of infusion status, real-time data collection, and timely alarms. It also uses mobile terminal assessment tools to remotely transmit pain assessment values and provides quality control management for postoperative analgesia, assisting departments in optimizing the quality management of postoperative pain relief.
2023 Revenue Summary: In 2023, Jiangsu Apon Medical’s revenue reached 1.52 million USD.

